Technology for preparing iron hydroxide with high specific surface area and cooperatively producing ammonium sulfate
A technology of iron oxyhydroxide and high specific surface, which is applied in iron oxide/iron hydroxide, oxidized water/sewage treatment, ammonia compounds, etc. It can solve the problems of cumbersome ammonium sulfate recovery process and small specific surface area of iron oxyhydroxide, and achieve The effects of large specific surface area, strong oxidation capacity, and strong adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment provides a process for preparing high specific surface iron oxyhydroxide and co-producing ammonium sulfate, comprising the following steps:
[0029] (1) Mix ferrous sulfate heptahydrate solid and ammonium carbonate solid with a molar ratio of 1:1.2 at 25°C, and add a certain amount of water to form a slurry during the kneading process, and control the density of the slurry during the kneading process. The pH value is 6.5-6.8, and the pH value of the slurry at the end of the kneading is 7.5-8;
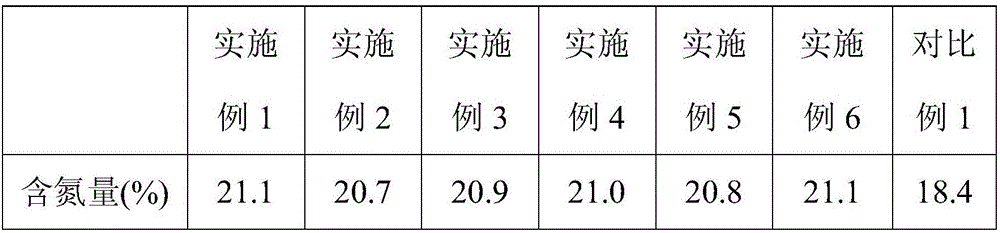
[0030] (2) carry out solid-liquid separation to the described slurry that step (1) obtains, collect solid phase and liquid phase respectively, described liquid phase is evaporated to dryness promptly to obtain ammonium sulfate;
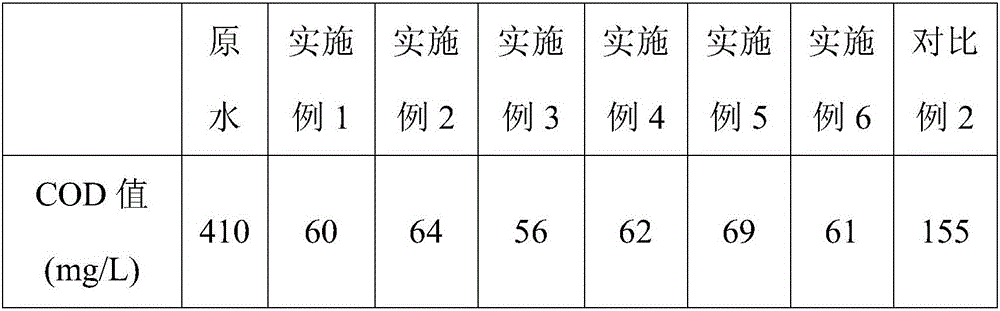
[0031] (3) Use water to make the solid phase into a slurry with a concentration of 15wt%, add 30wt% hydrogen peroxide to the slurry and control the molar ratio of hydrogen peroxide to ferrous sulfate to be 1:2 to have an oxidation reactio...
Embodiment 2
[0035] This embodiment provides a process for preparing high specific surface iron oxyhydroxide and co-producing ammonium sulfate, comprising the following steps:
[0036] (1) Mix ferrous sulfate heptahydrate solid and ammonium carbonate solid with a molar ratio of 1:1 at 20°C, and add a certain amount of water to form a slurry during the kneading process, and control the density of the slurry during the kneading process. The pH value is 6.7-7, and the pH value of the slurry at the end of kneading is 7.5-7.8;
[0037] (2) carry out solid-liquid separation to the described slurry that step (1) obtains, collect solid phase and liquid phase respectively, described liquid phase is evaporated to dryness promptly to obtain ammonium sulfate;
[0038] (3) adopting water to make the solid phase into a slurry with a concentration of 10wt%, adding 40wt% hydrogen peroxide to the slurry and controlling the mol ratio of hydrogen peroxide to ferrous sulfate to be 1.1:2 to have an oxidation r...
Embodiment 3
[0041] This embodiment provides a process for preparing high specific surface iron oxyhydroxide and co-producing ammonium sulfate, comprising the following steps:
[0042] (1) Mix ferrous sulfate heptahydrate solid and ammonium carbonate solid with a molar ratio of 1:1.1 at 30°C, and add a certain amount of water to form a slurry during the kneading process, and control the density of the slurry during the kneading process. The pH value is 6.6-6.9, and the pH value of the slurry at the end of the kneading is 7.6-8;
[0043] (2) carry out solid-liquid separation to the described slurry that step (1) obtains, collect solid phase and liquid phase respectively, described liquid phase is evaporated to dryness promptly to obtain ammonium sulfate;
[0044](3) Adopting water to make the solid phase into a slurry with a concentration of 20wt%, adding 50wt% hydrogen peroxide to the slurry and controlling the mol ratio of hydrogen peroxide to ferrous sulfate to be 1.05:2 to have an oxida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com