Method for purifying A-group and C-group meningococcus polysaccharides
A meningococcal and purification method technology, applied in the field of purification of group A group C meningococcal polysaccharide, can solve problems such as water body and air pollution, environmental hazards of phenol, etc., and achieve the effects of preventing damage, avoiding large-scale use and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
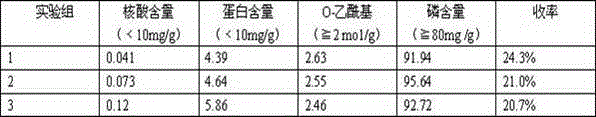
[0017] Purification of Group A Meningococcal Polysaccharide
[0018] Dissolve group A meningococcal jaggery in buffer (20mM Tris-HCl, 0.5% sodium deoxycholate, pH 8.0) at a concentration of 5-10mg / ml. After the dissolved sample was clarified and filtered through a 0.45 μm filter membrane, it was loaded on a DEAE-Sepharose Fast Flow chromatography column pre-equilibrated with buffer solution (20mM Tris-HCl, 0.5% sodium deoxycholate, pH 8.0), and the unadsorbed The flow-through peak was then loaded on a CaptoAdhere chromatographic column pre-equilibrated with buffer (20mM Tris-HCl, pH 8.0), eluted with 20mM Tris-HCl, pH 8.0 buffer as the mobile phase, and the polysaccharide-containing target peak. Then the collected polysaccharide solution is desalted with water for injection as the ultrafiltrate with an ultrafiltration membrane bag with a molecular weight cutoff of 300KD. The solution after desalination is the meningococcal polysaccharide solution of group A and group C, and ...
Embodiment 2
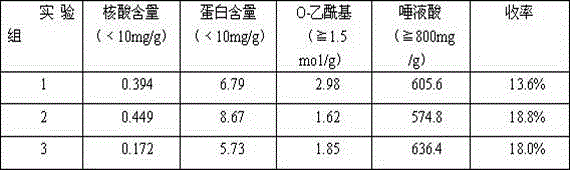
[0021] Preparation of Group C Meningococcal Polysaccharide
[0022] Dissolve group C meningococcal jaggery in buffer (20mM Tris-HCl, 0.5% sodium deoxycholate, pH 8.0) at a concentration of 6-8mg / ml. The dissolved sample was clarified and filtered through a 0.45 μm filter membrane, and loaded onto a DEAE-Sepharose Fast Flow column pre-equilibrated with buffer (20mM Tris-HCl, 0.5% sodium deoxycholate, pH 8.0). Adsorbed flow-through peaks were then loaded on a Capto Adhere chromatographic column pre-equilibrated with buffer (20mM Tris-HCl, pH 8.0), eluted with 20mM Tris-HCl, pH 8.0 buffer as the mobile phase, and collected Target peaks containing polysaccharides. Then the collected polysaccharide solution is desalted with water for injection as the ultrafiltrate with an ultrafiltration membrane bag with a molecular weight cutoff of 300KD. The solution after desalination is the meningococcal polysaccharide solution of group A and group C, and the quality index is tested by sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com