A crystalline form of a cyclin-dependent protein kinase inhibitor and a preparation method thereof
A crystallization and solvent technology, used in organic chemical methods, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of easy agglomeration, poor product stability, difficult filtration, etc., and achieve repeatable and controllable production processes and crystal stability. Good, stable production process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
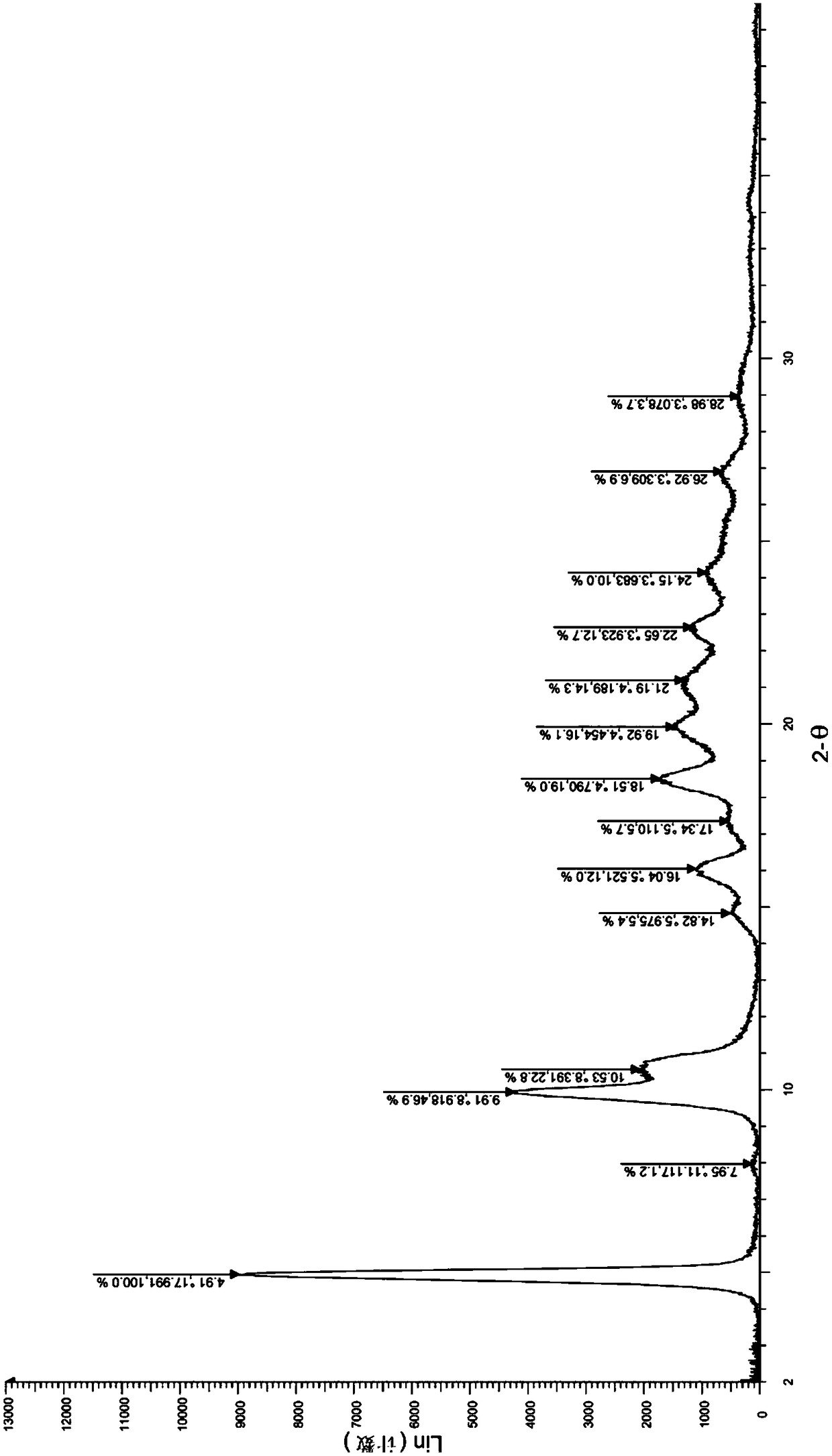
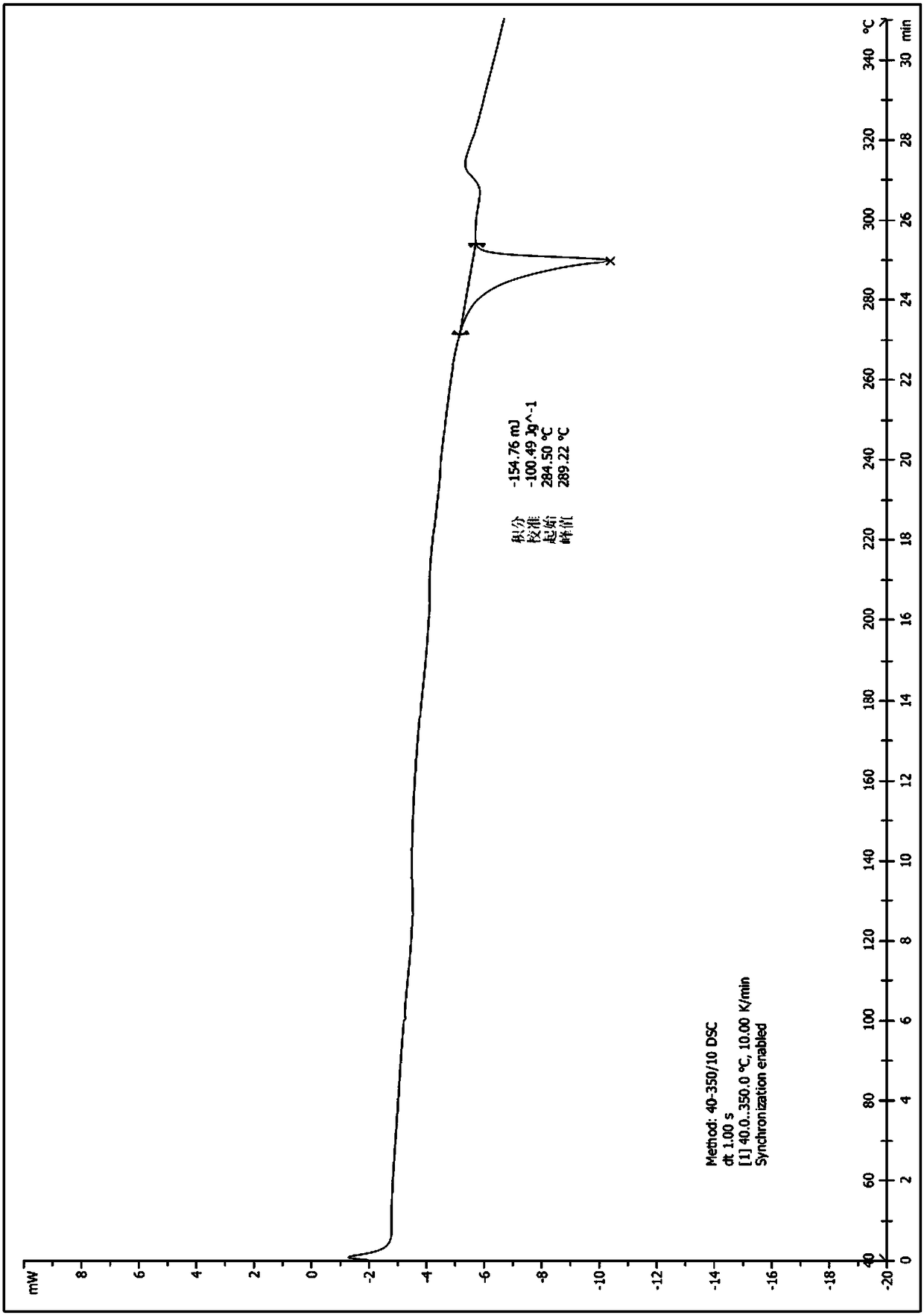
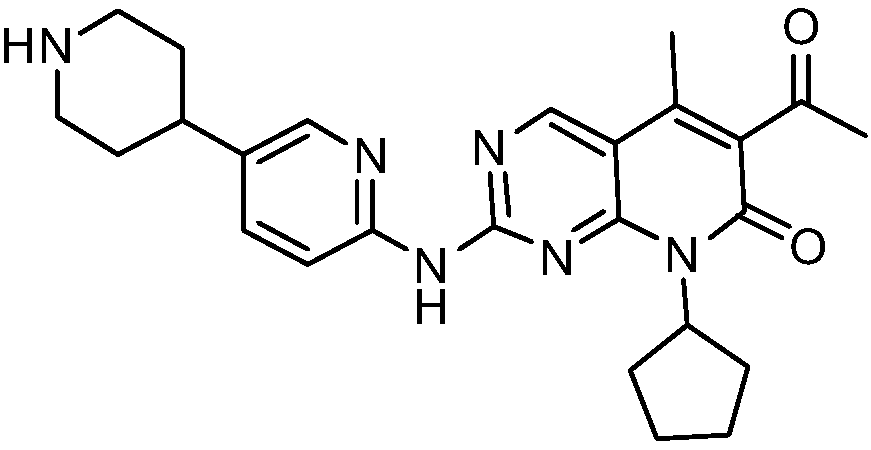
[0034] Take (1.0g, 2.24mmol) the compound represented by formula (I) (prepared according to the method provided by WO2014183520) and add it to a 250ml Erlenmeyer flask, add 40ml ethanol, stir at room temperature, then drop dilute hydrochloric acid (219mg, 6.01mmol) (dissolved in 4ml of water), heated to 60°C, dissolved, dropped into sodium bicarbonate solution (1.21g, 14.40mmol) (dissolved in 40ml of water), cooled to room temperature and stirred overnight. After drying, 0.89 g of solid was obtained, and the yield was 89.0%. The X-ray diffraction spectrum figure of this crystalline sample is shown in figure 1 . The crystallization at about 4.91 (17.99), 9.91 (8.92), 10.53 (8.39), 14.82 (5.98), 16.04 (5.52), 17.34 (5.11), 18.51 (4.79), 19.92 (4.45), 21.19 (4.19), 22.65 (3.92), 24.15(3.68), 26.92(3.31), and 28.98(3.08) have characteristic peaks. See the DSC spectrum figure 2 , with a sharp melting endothermic peak at 289.22°C, this crystal form is defined as I crystal form....
Embodiment 2
[0036] Get (1.0g, 2.24mmol) compound shown in formula (I) (prepared by Example 1) and join in the 250ml Erlenmeyer flask, add 40ml methanol, stir at room temperature, drop dilute hydrochloric acid (219mg, 6.01mmol) then ( dissolved in 4ml of water), heated to 60°C, dissolved, dropped into sodium bicarbonate solution (1.21g, 14.40mmol) (dissolved in 40ml of water), cooled to room temperature and stirred overnight. After drying, 0.98 g of solid was obtained, and the yield was 98.0%. Its X-diffraction and DSC patterns are researched and compared, and it is confirmed that the product is I crystal form.
Embodiment 3
[0038] Get (1.0g, 2.24mmol) the compound shown in formula (I) (prepared by Example 1) and join in the 250ml Erlenmeyer flask, add 40ml isopropanol, stir at room temperature, then drop dilute hydrochloric acid (219mg, 6.01mmol ) (dissolved in 4ml of water), heated to 60°C, dissolved, dropped into sodium bicarbonate solution (1.21g, 14.40mmol) (dissolved in 40ml of water), cooled to room temperature and stirred overnight. After drying, 0.52 g of solid was obtained, and the yield was 52.0%. Its X-diffraction and DSC patterns are researched and compared, and it is confirmed that the product is I crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com