The synthetic method of obeticholic acid
A technology of obeticholic acid and its synthesis method, which is applied in the field of synthesis technology of obeticholic acid, can solve the problems of low yield, immature synthesis route, harsh reaction conditions, etc., and achieve high cost, improved low temperature environment, and equipment less demanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、13
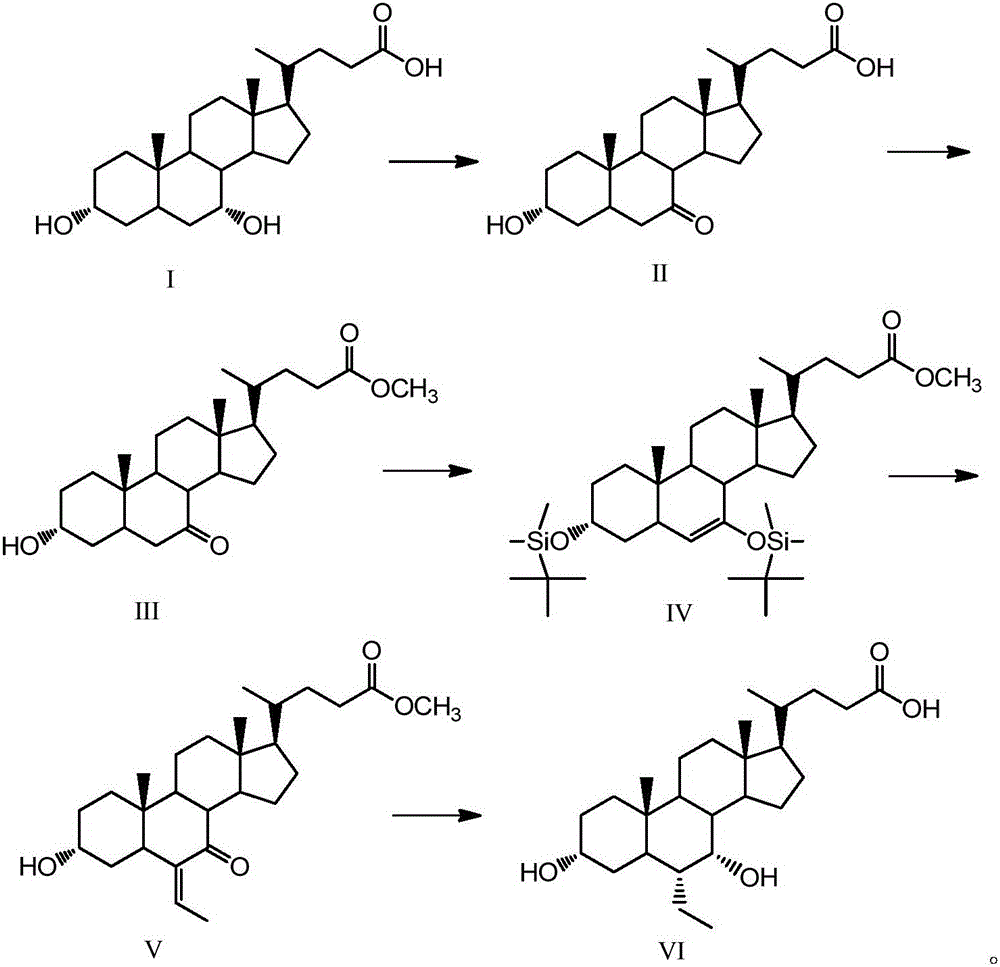
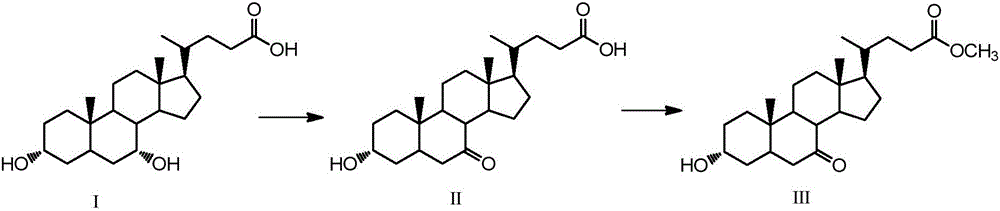
[0045] Embodiment 1, the synthesis of 13α-hydroxyl-7-ketone-5β-cholanic acid-24-methyl ester (Ⅲ)
[0046]Take 2g (5.1mmol) chenodeoxycholic acid (I), 1.3g (7.3mmol) N-bromosuccinimide in a 100mL single-necked round bottom flask, add 48mL acetone-water (3:1, volume ratio) , The reaction was stirred at room temperature for 4 h, and the reaction was detected by TLC. Add 10mL of 20% (mass fraction) sodium bisulfite to the flask, evaporate the acetone to dryness, then acidify with 5mL hydrochloric acid solution (5%), filter after solid precipitation, wash the filter residue with water until neutral, dry, and weigh with absolute ethanol After crystallization and drying, 1.84 g of white solid was obtained.
[0047] Take the obtained white solid in a 150mL single-necked round bottom flask, add 60mL of methanol, heat to 60°C, add dropwise 1mL of 98% concentrated sulfuric acid, reflux and stir for 12h, after the reaction is completed by TLC, add 2g of sodium bicarbonate, and wait until...
Embodiment 2、3
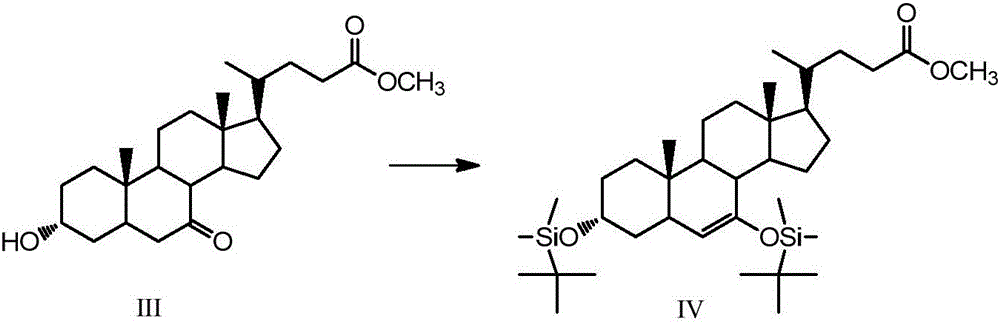
[0051] Example 2, 3α, the synthesis of 7-bis(tert-butyldimethylsilyloxy)-6-ene-5β-cholanic acid-24-methyl ester (Ⅳ)
[0052] Take 5g (12.4mmol) of compound (Ⅲ) in a 150mL single-necked round bottom flask, add 25mL of dry tetrahydrofuran and dissolve it, take 4.1g (27.3mmol) of tert-butyldimethylsilyl chloride and 2g (29.8mmol) of imidazole Add it into the constantly stirring solution, take 10mL triethylamine and slowly add it into the solution after 0.5h, and stir at room temperature for 4h after all the addition, and the reaction is completed by TLC detection. Add 25 mL of saturated sodium bicarbonate solution to the solution, and after the temperature of the solution returns to room temperature, extract the organic phase with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, and rotary evaporate to obtain 6.63 g of a brown oily solid that is the compound ( Ⅳ), yield 84%. 1 H NMR (CDCl 3 ,500MHz)δ:0~0.2(12H,m,3,7-OSi(t-Bu)Me 2 ), 0.66 (3H, s, 18-...
Embodiment 3
[0062] Embodiment 3, the synthesis of 3α-hydroxyl-6-ethylene-7-ketone-5β-cholanic acid-24-methyl ester (Ⅴ)
[0063] Take 12.7g (23.2mmol) of compound (Ⅳ) and dichloromethane (20mL) in a 150mL single-necked round bottom flask, cool down to -30°C, add 1.7mL (0.121mol) of paraldehyde and keep stirring, slowly drop Add 2.2mL (0.09mol) boron trifluoride diethyl ether, react for 1h under stirring, and then heat up to 0°C. Then add 20mL of dichloromethane to the solution, stir overnight, after TLC detects that the reaction is complete, add 6.5g (25mmol) tetrabutylammonium fluoride to the solution, after stirring for 1h, add 50mL of saturated sodium bicarbonate solution to the solution and then statically Place, extract the organic phase with dichloromethane, wash with saturated brine, dry over anhydrous sodium sulfate, distill under reduced pressure, and separate by flash column chromatography (petroleum ether: ethyl acetate = 7:3, v / v) to obtain a white solid 6.7 g is the compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com