GRPR targeted molecular probe and preparation method thereof
A molecular probe and targeting technology, applied in the field of GRPR targeting molecular probe and its preparation, can solve the problems of low tumor/background contrast, low tumor uptake rate, poor pharmacokinetics, etc. To achieve good pharmacokinetic properties and biological distribution characteristics, accurate test results, and ensure the effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
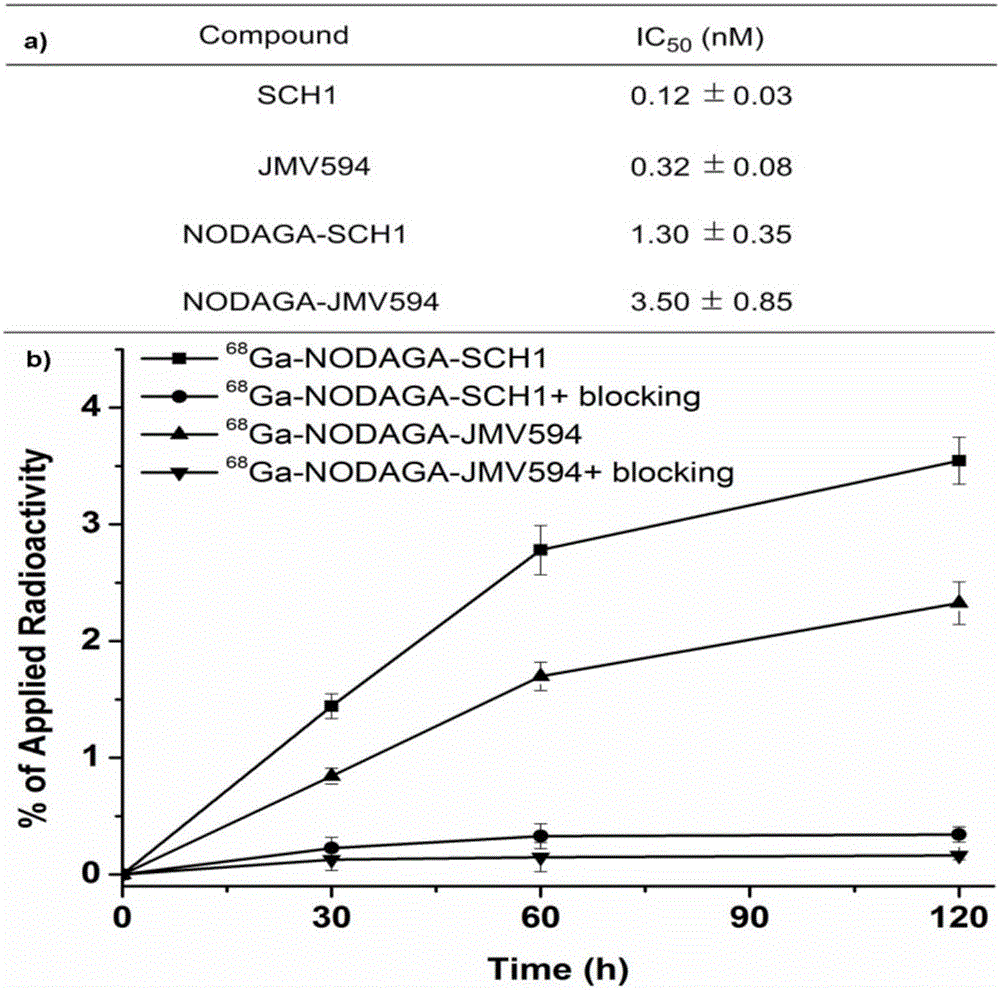
[0049] Embodiment 1: synthetic compound 68 Ga-NODAGA-JMV594
[0050]
[0051] The polypeptide JMV594 (1.13 mg, 0.01 mmol) and NODAGA-NHS ester (0.95 mg, 0.02 mmol, 2.0 equivalents) were weighed in a 2 mL test tube and dissolved in DMF solvent, and 2 equivalents of DIPEA were added. After 4 hours of reaction at room temperature. The reaction solution was directly purified by preparative HPLC and then vacuum freeze-dried to obtain a white solid powder NODAGA-JMV594, 0.80 mg, yield 55%. MS calculated value: C 70 h 103 N 17 o 18 + ([M+H] + ): 1470.8, measured value: ESI-MS: m / z 1470.8.
[0052] Dissolve 2nmol of NODAGA-JMV594 compound in 1mL NaOAc buffer (pH=4.5) and add 2mCi nuclides at 37°C 68 Ga labeling reaction 15min. labeled compound 68 After Ga-NODAGA-JMV594 was purified by HPLC, the organic solvent acetonitrile was blown dry with nitrogen gas, and diluted with PBS solution, it was directly used in cell uptake experiments and in vivo imaging experiments.
Embodiment 2
[0053] Embodiment 2: synthesis 68 Ga-NODAGA-SCH1
[0054]
[0055] Peptide SCH1 (1.25 mg, 0.01 mmol) and NODAGA-NHS ester (0.95 mg, 0.02 mmol, 2.0 equivalents) were weighed in a 2 mL test tube and dissolved in DMF solvent, and 2 equivalents of DIPEA were added. After 2 hours of reaction at room temperature. The reaction solution was directly purified by preparative HPLC and then vacuum freeze-dried to obtain a white solid powder NODAGA-SCH1, 1.30 mg, with a yield of 80%. MS calculated value: C 78 h 119 N 18 o 19 + ([M+H] + ): 1611.9, measured value: MALDI-TOF-MS: 1611.9.
[0056] Dissolve 2nmol of NODAGA-SCH1 compound in 1mL NaOAc buffer (pH=4.5) and add 2mCi nuclides at 37°C 68 Ga labeling reaction 15min. labeled compound 68 After Ga-NODAGA-SCH1 was purified by HPLC, the organic solvent acetonitrile was blown dry with nitrogen gas, and diluted with PBS solution, it was directly used in cell uptake experiments and in vivo imaging experiments.
Embodiment 3
[0057] Embodiment 3: synthetic compound 68 Ga-NODAGA-ZHS1
[0058]
[0059] Peptide ZH1 (1.28 mg, 0.01 mmol) and compound C1a (0.56 mg, 0.02 mmol, 2.0 equivalents) were weighed in a 2 mL test tube and dissolved in DMF solvent. After 2 hours of reaction at room temperature. The reaction solution was directly purified by preparative HPLC and then vacuum freeze-dried to obtain a white solid powder ZHS1, 1.13 mg, with a yield of 75%. MS calculated value: C 74 h 111 N 18 o 16 + ([M+H] + ): 1506.8, measured value: ESI-MS: 1506.9.
[0060] Weigh the polypeptide ZHS1 (1.56mg, 0.01mmol) and NODAGA-NHS ester (0.95mg, 0.02mmol, 2.0eq) in a 2mL test tube and dissolve them in DMF solvent, and add 2equivalents of DIPEA. After 4 hours of reaction at room temperature. The reaction solution was directly purified by preparative HPLC and then vacuum freeze-dried to obtain a white solid powder NODAGA-ZHS1, 1.50 mg, with a yield of 80%. MS calculated value: C 89 h 134 N 21 o 23 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com