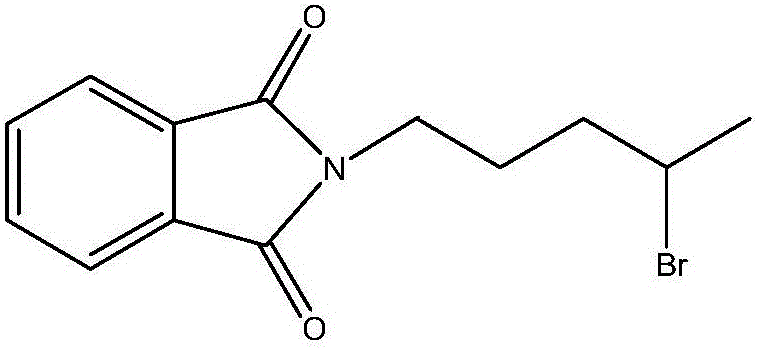
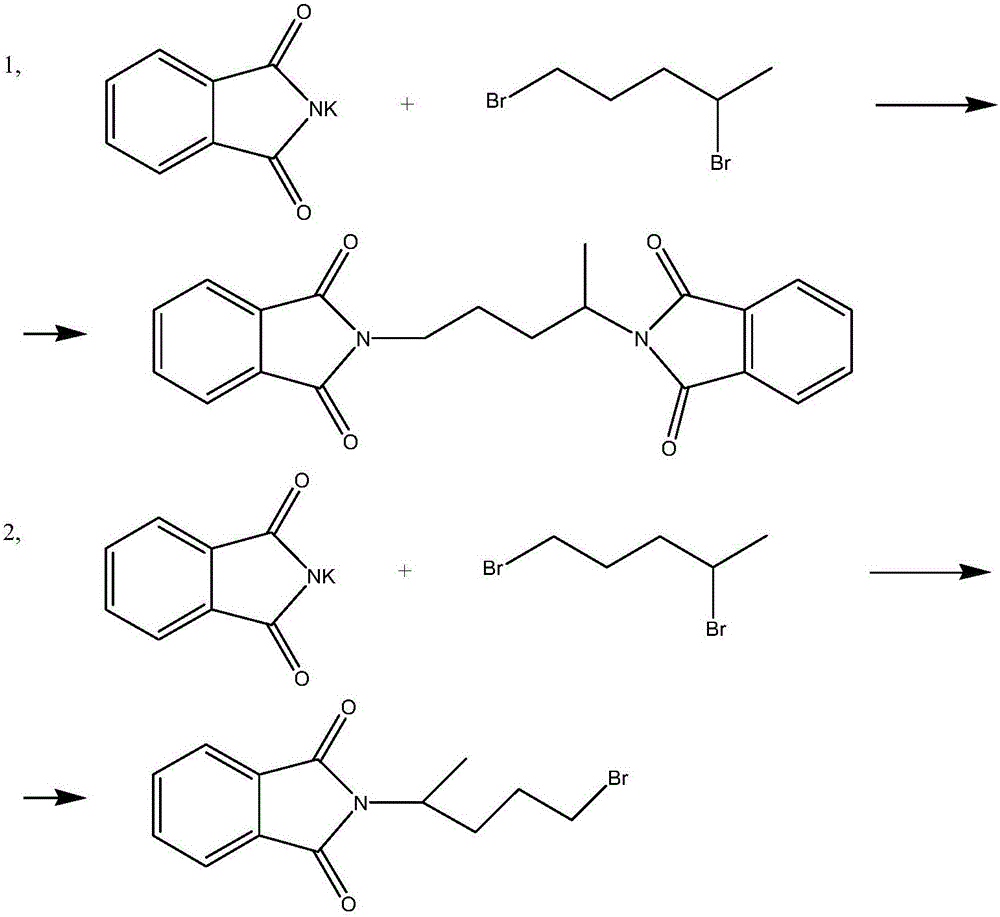
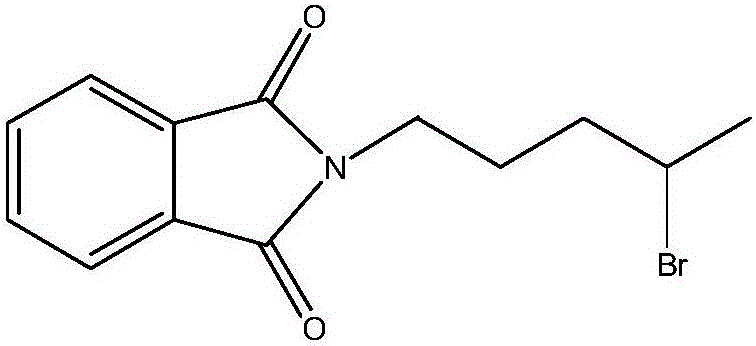
Synthesis method of antimalarial drug primaquine phosphate intermediate N-(4-bromopentyl)phthalimide
A technology of phthalimide and primaquine phosphate, applied in a new synthesis field, can solve problems such as inability to adopt purification, inability to adopt recrystallization purification, etc., and achieves low cost, high conversion rate, and reaction operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1-1
[0047] In a 1000ml four-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, 50g (0.48mol) 5-chloro-1-pentene, 50g (0.27mol) phthalimide potassium salt, 2.5g triphenylmethylphosphine chloride, 0.5g cobalt acetate and 300g N,N-dimethylformamide. After the addition, the oil bath was heated to reflux at a temperature of 152° C. and kept for 8 hours.
[0048] After keeping warm, cool to room temperature. Filtration, the filter cake is the reaction by-product potassium chloride, polymerization inhibitor and catalyst. Rinse the filter cake with 100ml of fresh DMF, and combine the filtrate and washings. DMF and excess 5-chloro-1-pentene were evaporated under reduced pressure with a rotary evaporator. 54.5 g of light yellow viscous liquid was obtained, the content measured by liquid chromatography was 98.25%, and the yield was 93.79%.
example 1-2
[0050] In a 1000ml four-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, 50g (0.48mol) 5-chloro-1-pentene, 50g (0.27mol) phthalimide potassium salt, 0.25g of triphenylmethylphosphine bromide, 2.5g of nickel sulfate and 500g of hexamethylphosphonic triamide. After the addition, the oil bath was heated to 200° C., and the reaction was kept for 3 hours.
[0051] After keeping warm, cool to room temperature. Filtration, the filter cake is the reaction by-product potassium chloride, polymerization inhibitor and catalyst. Rinse the filter cake with 100ml of fresh acetone, and combine the filtrate and washings. Acetone, hexamethylphosphonic triamide and excess 5-chloro-1-pentene were evaporated under reduced pressure with a rotary evaporator. 57g of red viscous liquid was obtained, the content measured by liquid chromatography was 94.81%, and the yield was 98.09%.
example 1-3
[0053] In a 2000ml four-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, 200g (1.91mol) of 5-chloro-1-pentene, 200g (1.08mol) of phthalimide potassium salt, 5g of triphenylethylphosphine chloride, 4g of nickel sulfate, 4g of cobalt acetate and 800g of hexamethylphosphonic triamide. After the addition, the oil bath was heated to 100° C., and the reaction was kept for 8 hours.
[0054] After the heat preservation is finished, follow the post-treatment operation of Example 1-2 to obtain 224 g of light yellow viscous liquid, the content of which is 99.46% as measured by liquid chromatography, and the yield is 96.37%.
[0055] ②, the synthesis of N-(4-bromopentyl)phthalimide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com