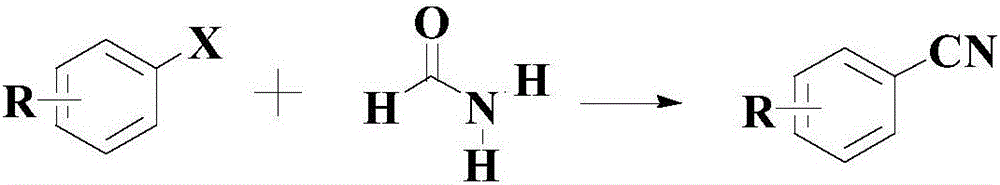
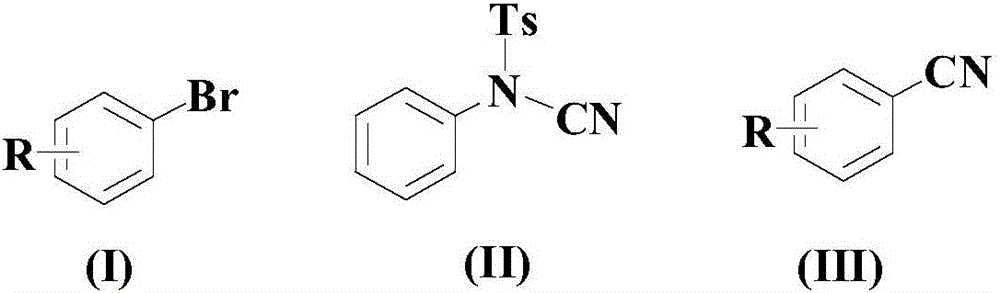
Synthetic method for cyanophenyl compound
A synthesis method and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. The effect of good industrial production prospects and potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] At room temperature, in an appropriate amount of organic solvent PEG-200, add 100mmol of the above formula (I) compound, 150mmol of the above formula (II) compound, 20mmol of catalyst (5mmol bis(1,5-cyclooctadiene) iridium tetrafluoroborate and 15mmol AgSbF 6 mixture), 20mmol ligand L1 and 15mmol auxiliary agent (a mixture of 7.5mmol lanthanum trifluoromethanesulfonate and 7.5mmol cerium ammonium nitrate), then the temperature was raised to 80°C, and the reaction was stirred at this temperature for 12 hours;
[0036] After the reaction is over, filter the reaction system while it is hot, add an equal volume of saturated sodium carbonate aqueous solution to the filtrate, shake fully, then add ethyl acetate to extract 2-3 times, combine the organic phases, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure , the residue was separated by 300-400 mesh silica gel column chromatography, and eluted with a mixture of acetone and petroleum e...
Embodiment 2
[0039]
[0040] At room temperature, in an appropriate amount of organic solvent PEG-200, add 100mmol of the above formula (I) compound, 250mmol of the above formula (II) compound, 10mmol of catalyst (2mmol bis(1,5-cyclooctadiene) iridium tetrafluoroborate and 8 mmol AgSbF 6 mixture), 30mmol ligand L1 and 8mmol auxiliary agent (a mixture of 4mmol lanthanum trifluoromethanesulfonate and 4mmol cerium ammonium nitrate), then the temperature was raised to 100°C, and the reaction was stirred at this temperature for 8 hours;
[0041]After the reaction is over, filter the reaction system while it is hot, add an equal volume of saturated sodium carbonate aqueous solution to the filtrate, shake fully, then add ethyl acetate to extract 2-3 times, combine the organic phases, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure , the residue was separated by 300-400 mesh silica gel column chromatography, and eluted with a mixture of acetone and petroleum ether a...
Embodiment 3
[0044]
[0045] At room temperature, in an appropriate amount of organic solvent PEG-200, add 100mmol of the above formula (I) compound, 200mmol of the above formula (II) compound, 15mmol of catalyst (3.5mmol bis(1,5-cyclooctadiene) iridium tetrafluoroborate with 11.5mmol AgSbF 6 mixture), 25mmol of ligand L1 and 12mmol of additives (a mixture of 6mmol of lanthanum trifluoromethanesulfonate and 6mmol of cerium ammonium nitrate), then the temperature was raised to 90°C, and the reaction was stirred at this temperature for 10 hours;
[0046] After the reaction is over, filter the reaction system while it is hot, add an equal volume of saturated sodium carbonate aqueous solution to the filtrate, shake fully, then add ethyl acetate to extract 2-3 times, combine the organic phases, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure , the residue was separated by 300-400 mesh silica gel column chromatography, and eluted with a mixture of acetone and petr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com