Preparation method for adriamycin-loaded polyethyleneimine-hyaluronic acid-modified hectorite-coated gold nanoparticles
A technology of hyaluronic acid modification and polyethyleneimine, applied in the field of preparation of drug-loaded nanomaterials, to increase the circulation time in the body, sensitive CT imaging effect, and realize the effect of targeted therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
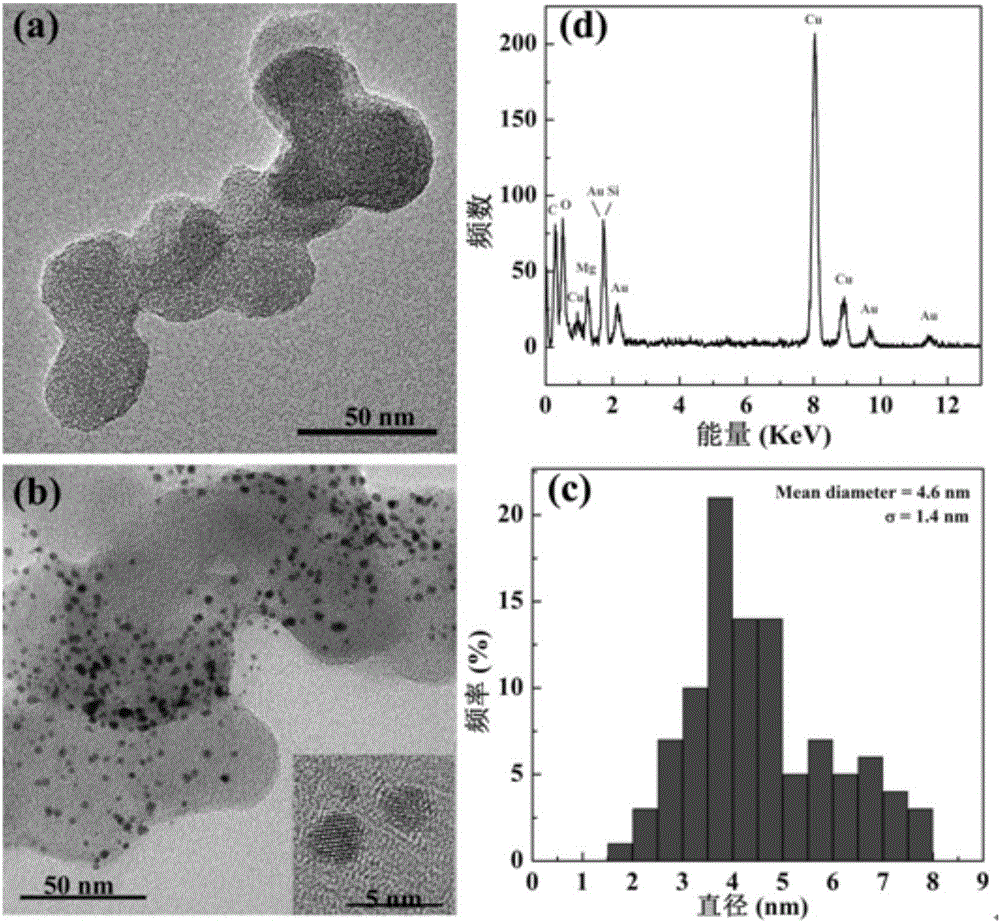
Image
Examples
Embodiment 1
[0083] (1) In 50 mL PLA-PEG-COOH mixed solution of acetone and water (6 mg / mL), 10 mL LAP aqueous solution (10 mg / mL) was slowly added dropwise, and the reaction was stirred for 1 hour. After the reaction, all the obtained solution was transferred to a dialysis bag with a cutoff analysis volume of 8000-14000. Put the dialysis bag into a beaker filled with 2L deionized water for dialysis. The dialysis lasts for 2-4 days, and the water is changed 3-4 times a day. After the dialysis, the product in the dialysis bag was transferred to a 100mL glass bottle and stored at 4°C to obtain polyethylene glycol-modified LAP-PLA-PEG-COOH.
[0084] (2) Take the above 30mL LAP-PLA-PEG-COOH aqueous solution (3mg / mL), add 2mL EDC aqueous solution (8.42mg / mL), stir and react for 3 hours. Then it was added dropwise to 10.98 mL of PEI in water (1 mg / mL), and reacted with rapid stirring for 3 days. After the reaction, all the obtained solution was transferred to a dialysis bag with a cut-off anal...
Embodiment 2
[0087] Analyze the stability of the material with the ultraviolet-visible absorption spectrum, take 0.5mg / mL of the functionalized dendrimer material prepared in Example 1, the solvent is distilled water, and place it under the temperature conditions of 4°C, 37°C, and 50°C Under the conditions of pH=5, 6, 7, 8, measure its ultraviolet-visible absorption spectrum.
Embodiment 3
[0089] Get the product gained in Example 1 and use distilled water as a solvent to prepare a mother liquor with a gold concentration of 0.04M. Afterwards, 0.02, 0.01, 0.005, 0.0025 and 0.005M samples were serially diluted. At the same time, using iohexol for clinical use as a reference material, samples with corresponding iodine concentrations were diluted with distilled water. Afterwards, CT imaging tests were performed on the two sets of materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com