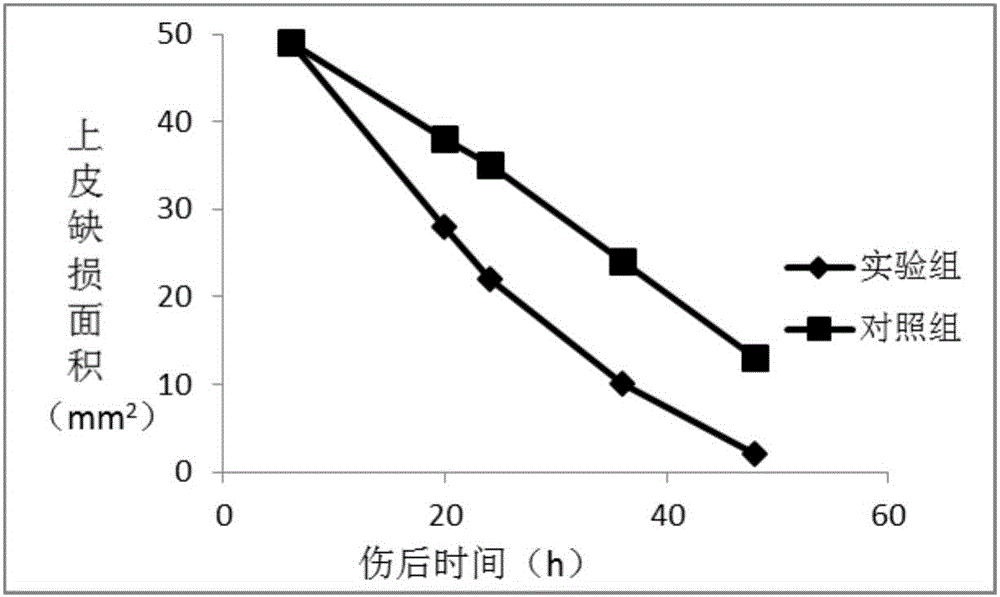
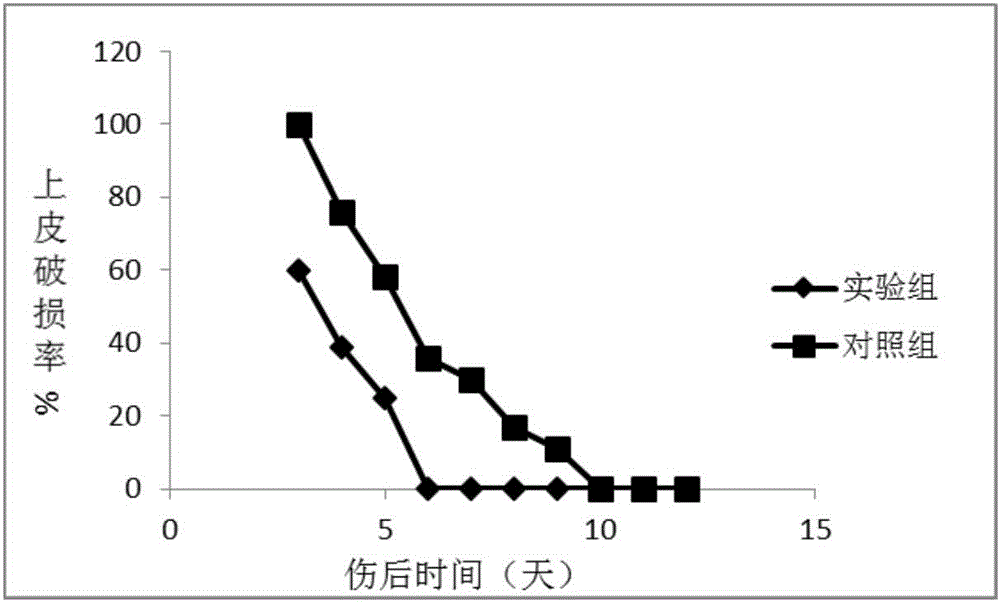
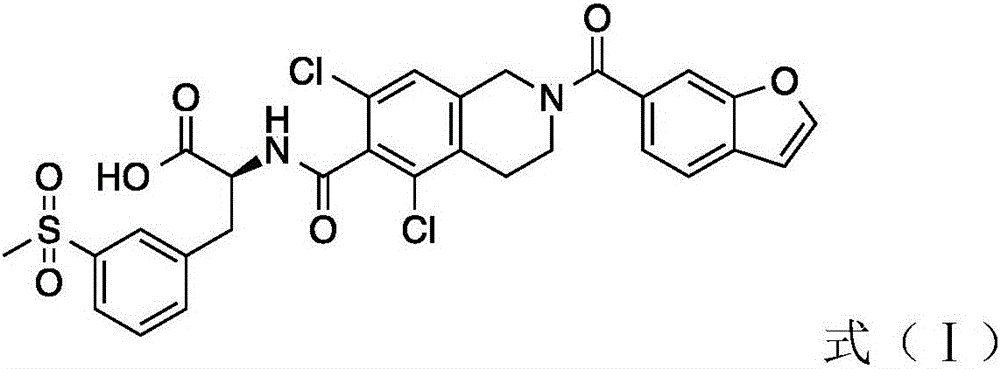
Extracellular matrix imitated eye drops and preparation method thereof
A technology of eye drops and extracellular matrix, which is applied in the field of medicine, can solve the problems of interfering with the normal metabolism of corneal epithelium, reducing the clinical curative effect, aggravating the disease, etc., and achieves the effect of prolonging the drug action time, simple preparation method, and high-efficiency drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Eye drop formula
[0033]An eye drop imitating extracellular matrix, which comprises the following components in parts by weight: 8 parts of Lifitegrast, 2 parts of heparan sulfate of 0.5mg / ml, 6 parts of sodium hyaluronate of 150wDa, 0.06 parts of growth factor of 10000IU / ml, 0.5 parts of collagen and 300 parts of water for injection.
[0034] The pH of the eye drops is 7.5, which is adjusted by boric acid buffer.
[0035] The osmotic pressure of the eye drops is 300mOsm / L, adjusted by sodium chloride and potassium chloride, the mass concentration of sodium chloride is 0.5%, and the mass concentration of potassium chloride is 0.1%.
[0036] Heparan sulfate is extracted from natural ingredients or produced by bacterial fermentation; sodium hyaluronate is produced by cockscomb extraction or fermentation.
[0037] The growth factor is prepared by using epidermal growth factor: fibroblast growth factor: basic fibroblast growth factor: placenta growth factor according ...
Embodiment 2
[0044] 1. Eye drop formula
[0045] An eye drop imitating extracellular matrix, which comprises the following components in parts by weight: 5 parts of Lifitegrast, 0.1 part of heparan sulfate of 30mg / ml, 4 parts of sodium hyaluronate of 40wDa, 0.1 part of growth factor of 4000IU / ml, collagen 0.1 part of protein and 200 parts of water for injection.
[0046] The pH of the eye drops is 5.5, adjusted with phosphate buffer.
[0047] The osmotic pressure of the eye drops is 350mOsm / L, adjusted by sodium chloride, and the mass concentration of sodium chloride is 0.3%.
[0048] Heparan sulfate is extracted from natural ingredients or produced by bacterial fermentation; sodium hyaluronate is produced by cockscomb extraction or fermentation.
[0049] The growth factor is prepared by using epidermal growth factor: fibroblast growth factor: basic fibroblast according to the weight ratio of 1:3:1.
[0050] 2. Preparation method of eye drops
[0051] (1) First take by weighing the sod...
Embodiment 3
[0056] 1. Eye drop formula
[0057] An eye drop imitating extracellular matrix, which comprises the following components in parts by weight: 10 parts of Lifitegrast, 3 parts of heparan sulfate of 30mg / ml, 1 part of sodium hyaluronate of 100wDa, 0.01 part of growth factor of 5000IU / ml, collagen 1 part protein and 500 parts water for injection.
[0058] The pH of the eye drops is 6, which is adjusted by phosphate buffer and boric acid buffer.
[0059] The osmotic pressure of the eye drops is 280mOsm / L, adjusted by potassium chloride, and the mass concentration of potassium chloride is 0.1%.
[0060] Heparan sulfate is extracted from natural ingredients or produced by bacterial fermentation; sodium hyaluronate is produced by cockscomb extraction or fermentation.
[0061] The growth factor is prepared by using epidermal growth factor: basic fibroblast growth factor: placental growth factor according to the weight ratio of 1:4:3.
[0062] 2. Preparation method of eye drops
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com