Compound anisodamine and neostigmine sustained-release tablet and preparation method thereof
A technology of anisodamine and neostigmine, applied in the field of compound anisodamine neostigmine sustained-release tablets and its preparation, can solve the problems of short half-life and inability to meet anti-inflammatory treatment, and reduce the peak concentration , large application value, and the effect of increased action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of Compound Anisodamine Neostigmine Sustained Release Tablets
[0049] 1. The formula of sustained-release tablet (according to 1000 tablets): by weight, anisodamine 16.67 parts, neostigmine: 0.03 parts, HPMCK4M: 20 parts, magnesium stearate: 0.7 parts, polyvinylpyrrolidone K30: 15 parts, pregelatinized starch: 8 parts, microcrystalline cellulose: 39.6 parts.
[0050] 2. The preparation method of the sustained-release tablet:
[0051] (1) Raw material preparation: pass anisodamine, neostigmine, HPMC K4M, pregelatinized starch, and microcrystalline cellulose through a 100-mesh sieve for later use;
[0052] (2) Mixing: Mix the sieved anisodamine, neostigmine, HPMC K4M, pregelatinized starch, and microcrystalline cellulose for 30 minutes to make drug fine powder for later use;
[0053] (3) Wet granulation: Dissolve polyvinylpyrrolidone K30 in distilled water, mix with the above-mentioned drug fine powder, granulate with a 20-mesh nylon screen, a...
Embodiment 2
[0056] Embodiment 2: Preparation of Compound Anisodamine Neostigmine Sustained Release Tablets
[0057] 1. Prescription for sustained-release tablets (calculated as 1000 tablets): by weight, anisodamine: 16.67 parts, neostigmine: 0.03 parts, HPMCK4M: 15 parts, magnesium stearate: 0.7 parts, polyvinylpyrrolidone K30: 15 parts, pregelatinized starch: 8 parts, microcrystalline cellulose: 44.6 parts.
[0058] 2. The preparation method of the sustained-release tablet:
[0059] (1) Raw material preparation: pass anisodamine, neostigmine, HPMC K4M, pregelatinized starch, and microcrystalline cellulose through a 100-mesh sieve for later use;
[0060] (2) Mixing: Mix the sieved anisodamine, neostigmine, HPMC K4M, pregelatinized starch, and microcrystalline cellulose for 30 minutes to make drug fine powder for later use;
[0061] (3) Wet granulation: Dissolve polyvinylpyrrolidone K30 in distilled water, mix with the above-mentioned drug fine powder, granulate with a 20-mesh nylon scre...
Embodiment 3
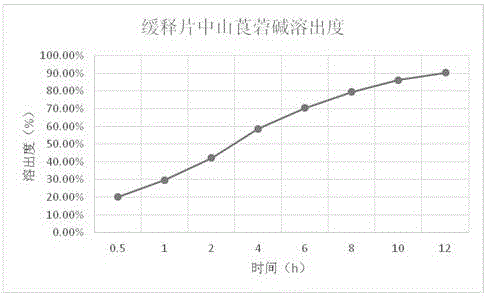
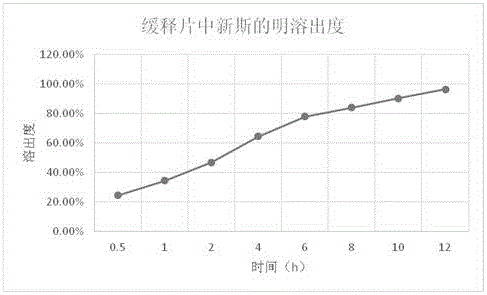
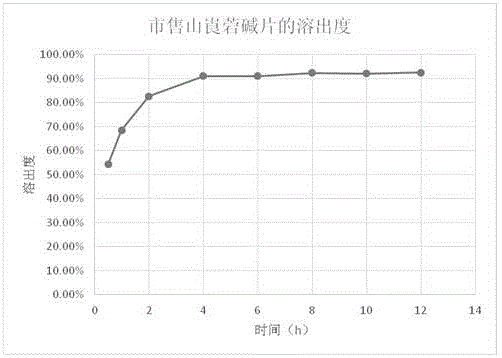
[0064] Example 3: Research on the Dissolution of Anisodamine in Compound Anisodamine Neostigmine Sustained Release Tablets
[0065] Refer to the dissolution test method of anisodamine tablets ("Chinese Pharmacopoeia" 2015 edition), adopt the paddle method, use 900ml of 0.1mol / L hydrochloric acid solution as the dissolution medium, rotate at 50 rpm, and set the temperature at 37°C to measure the dissolution Dissolution after 1, 2, 4, 8, 10, 12 hours. The method is to take 10ml of the solution at each time point, filter, and supplement the dissolution medium with a 0.1mol / L hydrochloric acid solution with the same volume and temperature as the taken solution, accurately measure 20ul of the subsequent filtrate, inject it into the liquid chromatograph, and use octadecyl Silane-bonded silica gel was used as filler, 0.01mol / L potassium dihydrogen phosphate solution (containing 0.15% triethylamine, pH value adjusted to 6.5 with phosphoric acid)-methanol (70:30) was used as mobile phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com