Scandium recovery method
A recovery method and process technology, which is applied to the field of high-efficiency recovery of scandium contained in nickel oxide ore, and can solve the problems of low grade nickel content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] [Leaching process S1]
[0121]First, nickel oxide ore and concentrated sulfuric acid are put into an autoclave, and a slurry containing valuable metals such as scandium and nickel is generated at 245°C for 1 hour, and solid-liquid separation from the slurry contains various The leaching liquid and leaching residue of valuable metals.
[0122] [Neutralization process S2]
[0123] Calcium carbonate is then added to the leach liquor to obtain a neutralized precipitate and a neutralized liquor. The neutralized liquid contains valuable metals such as scandium and nickel, and the neutralized precipitate contains most of the impurities represented by aluminum.
[0124] [Vulcanization process S3]
[0125] Next, hydrogen sulfide gas is blown into the neutralized liquid to form sulfides from nickel, cobalt, and zinc, which are separated from the sulfided liquid.
[0126] [Ion exchange step S4]
[0127] [Adsorption step S41]
[0128] Slaked lime was added as a neutralizing a...
Embodiment 2
[0175] The same scandium oxide ore as in Example 1 above was leached, neutralized, and vulcanized by the same method as in Example 1 above to obtain a sulfide liquid with the composition shown in Table 7.
[0176] Composition of liquid after table 7 vulcanization
[0177] sc
Al
Fe
Th
U
14
2800
1000
0.2
0.7
[0178] (unit is mg / L)
[0179] Carry out ion exchange process and concentration process with the method identical with above-mentioned embodiment 1, redissolve the obtained scandium hydroxide with sulfuric acid, obtain chelating eluent hydroxide solution. Table 8 shows the results of analyzing the composition of the chelate eluent hydroxide solution.
[0180] The composition of table 8 chelating eluent hydroxide solution
[0181] sc
Al
Fe
Th
U
20
11
4
140
220
[0182] (unit is mg / L)
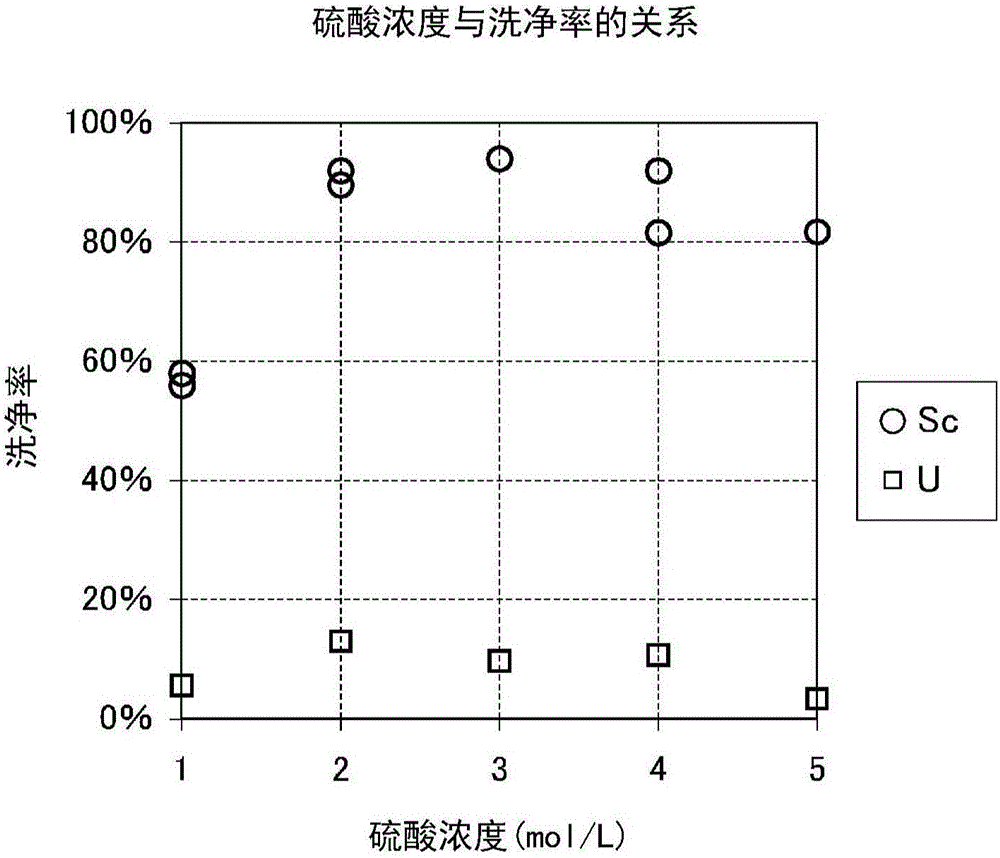
[0183] The chelated eluent hydroxide solution having the composition shown in Table 8 was us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com