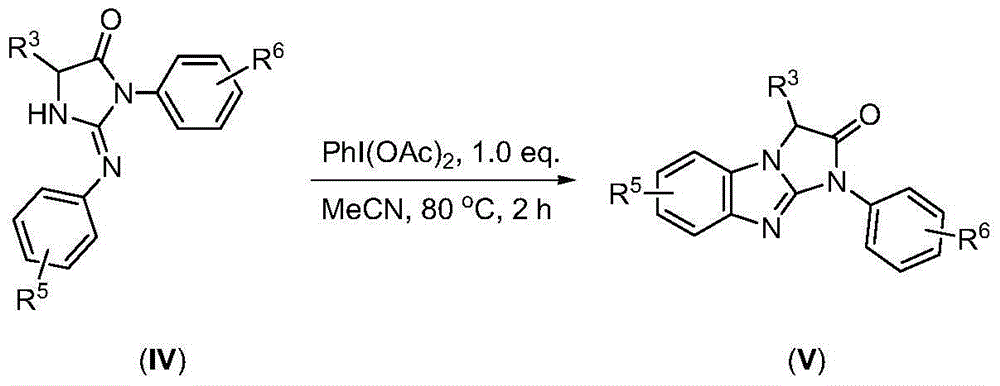
Synthesis method and application of imidazole derivative
A synthesis method and derivative technology, applied in the direction of organic chemistry, etc., can solve the problems of poor tolerance of functional groups, complex and difficult to obtain raw materials, long reaction time, etc., and achieve the effect of scientific and reasonable synthesis method, convenient development and application, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
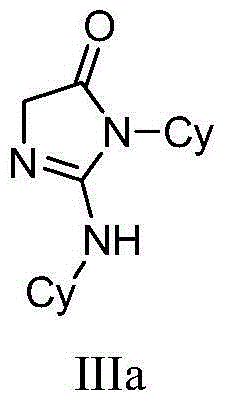
[0040] Embodiment 1——compound shown in preparation formula IIIa (R 1 =R 2 =Cy, R 3 =R 4 =H):
[0041]
[0042]At room temperature, add 1.05 mmol of ethyl glycine hydrochloride, 1.05 mmol of triethylamine and 0.05 mmol of zinc trifluoromethanesulfonate into a 25 mL reaction tube, and add 5 mL of benzene to dissolve. Then add 1.0 mmol of N,N'-dicyclohexylcarbodiimide, and react at 80°C for 8 hours. The reaction system is a yellow suspension. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether:ethyl acetate=6:1 was used as an eluent to obtain 239 mg of imidazolone derivative (IIIa) (purity>98%, yellow solid), and the isolated yield was 91%.
[0043] The NMR data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 ): δ1.24(d, J=6.2Hz, 6H, CH 3 ), 1.42, (d, J=7.0Hz, 6H, CH 3 ),3.92(s,2H,CH 2 ),4.13-4.25(m,2H,CH); 13 C NMR (100MHz, CDCl 3 ): δ19.73, 22.85, 43.56, 55.76, 60.09, 155.35, 1...
Embodiment 2
[0044] Embodiment 2——compound shown in preparation formula IIIb (R 1 =R 2 = 4-MeC 6 h 4 , R 3 =R 4 =H):
[0045]
[0046] At room temperature, add 1.05 mmol of ethyl glycine hydrochloride, 1.05 mmol of triethylamine and 0.05 mmol of zinc trifluoromethanesulfonate into a 25 mL reaction tube, and add 5 mL of benzene to dissolve. Then add 1.0 mmol of N,N'-di-p-methylphenylcarbodiimide, and react at 80°C for 8 hours. The reaction system is a yellow suspension. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether: ethyl acetate=6:1 was used as an eluent to obtain 262 mg of imidazolone derivative (IIIb) (purity>98%, yellow solid), and the isolated yield 94%.
[0047] The NMR data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 ):δ2.29(s,3H,CH 3 ),2.38(brs,3H,CH 3 ),3.90(brs,1H,CH 2 ),4.22(brs,1H,CH 2 ),4.38(brs,1H,NH),6.81-6.83(m,2H,CH),7.07-7.09(m,2H,CH),7.24-7.29(m,4H,CH); 13 C N...
Embodiment 3
[0048] Embodiment 3——compound shown in preparation formula IIIc (R 1 =Bn,R 2 =2,6- i PrC 3 h 6 , R 3 =R 4 =H):
[0049]
[0050] At room temperature, add 1.05 mmol of ethyl glycine hydrochloride, 1.05 mmol of 4-(N,N-dimethylamino)pyridine and 0.05 mmol of zinc trifluoromethanesulfonate to a 25 mL reaction tube, add 5 mL of benzene dissolve. Then 1.0 mmol of 1-benzyl-3-(2,6-diisopropylphenyl)diimine was added and reacted at 50° C. for 4 hours. The reaction system is a yellow suspension. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether: ethyl acetate=6:1 was used as an eluent to obtain 255 mg of imidazolone derivative (IIIc) (purity>98%, yellow solid), and the isolated yield was 71%.
[0051] The NMR data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 ):δ1.08-1.10(m,12H,CH 3 ),2.82-2.89(m,2H,CH),3.85(s,2H,CH 2 ),4.25(brs,1H,NH),4.91(s,2H,CH 2 ),7.00-7.10(m,3H,CH),7.27-7.34...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com