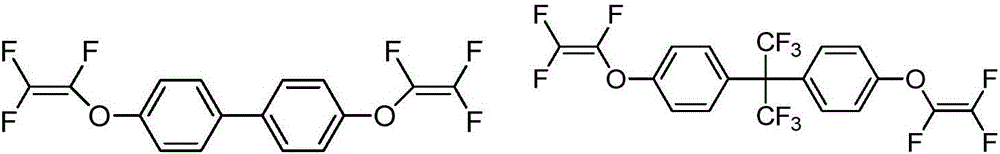
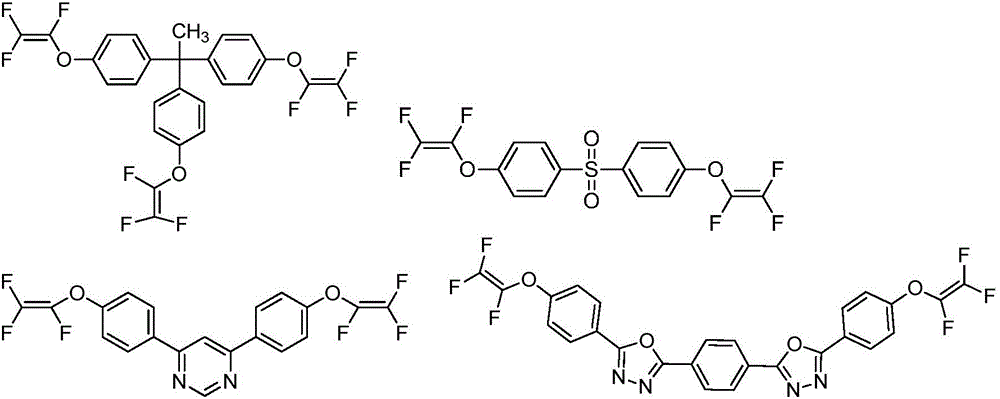
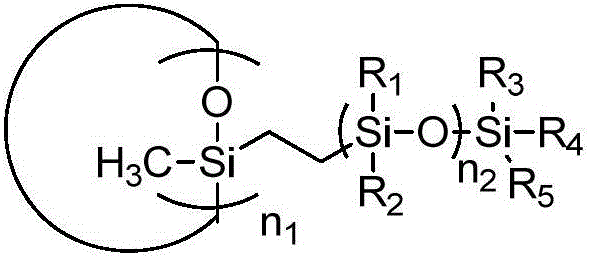
Preparation and application of trifluorovinyl-ether-containing cyclosiloxane capable of direct heat curing
A technology of trifluorovinyl ether and cyclosiloxane, applied in the direction of silicon organic compounds, etc., can solve the problems of limited application, low mechanical properties, complex multi-component reaction process, etc., and achieve great application prospects and cross-linking degree. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] Preparation of Cyclosiloxane
[0082] The present invention also provides a method for preparing the cyclosiloxane, the method comprising the steps of: reacting the following monomer 1 and monomer 2 to obtain the cyclosiloxane as described in the first aspect of the present invention ;
[0083]
[0084] Or react with monomer 3 and monomer 4 as shown below to obtain cyclosiloxane as described in the first aspect of the present invention
[0085]
[0086] Wherein, the definition of each group is as described in the first aspect of the present invention.
[0087] The cyclic siloxane structure part is connected with the silane segment part containing trifluorovinyl ether phenyl group through hydrogen addition reaction. Preferably, the reaction is carried out in the presence of a catalyst selected from the group consisting of chloroplatinic acid, chloroplatinic acid-isopropanol solution, methylvinylsiloxane platinum complex, Karstedt catalyst, palladium complex, Rho...
Embodiment 1
[0127] Example 1 Preparation of 4-trifluorovinyl ether phenyldimethylsilane
[0128]
[0129] Under the protection of nitrogen, add 4.10 grams of magnesium strips, 26.08 grams of dimethylchlorosilane, a small amount of iodine and 100 milliliters of dry treated tetrahydrofuran into a dry 250 milliliter three-necked flask, stir and slowly add 35.10 grams of 4- Dissolve trifluorovinyl ether bromobenzene in 20 ml of tetrahydrofuran solution, heat slightly until the color of the system fades, react at room temperature for 15 hours after the dropwise addition, remove the solvent after filtering with diatomaceous earth, and collect the fraction 23.8 at 80°C / 20mba by distillation under reduced pressure g, yield 74%. 1 H NMR (400MHz, CDCl 3 ,δin ppm): 7.58~7.60(d,2H), 6.89~6.91(d,2H), 4.41-4.45(m,H), 1.20~1.24(t,6H). 19 F-NMR (376MHz, CDCl3, δin ppm): -119.35, -119.50, -119.60, -119.76, -126.15, -126.41, -126.44, -126.70, -133.56, -133.72, -133.72, -133.86, -134.01 .
Embodiment 2
[0130] Example 2 Preparation of 4-trifluorovinyl ether phenyldimethylchlorosilane
[0131] Under the protection of nitrogen, add 4.05 grams of magnesium strips, 34.85 grams of dimethyldichlorosilane, a small amount of iodine and 150 milliliters of dry treated tetrahydrofuran into a dry 250 milliliter three-necked flask, stir and slowly add 34.20 grams of 4 - Dissolve trifluorovinyl ether bromobenzene in 20 ml of tetrahydrofuran solution, heat slightly until the color of the system fades, react at room temperature for 20 hours after the dropwise addition, filter with diatomaceous earth, remove the solvent, and collect the fraction at 85°C / 20mba by vacuum distillation 22.47 g, 65% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com