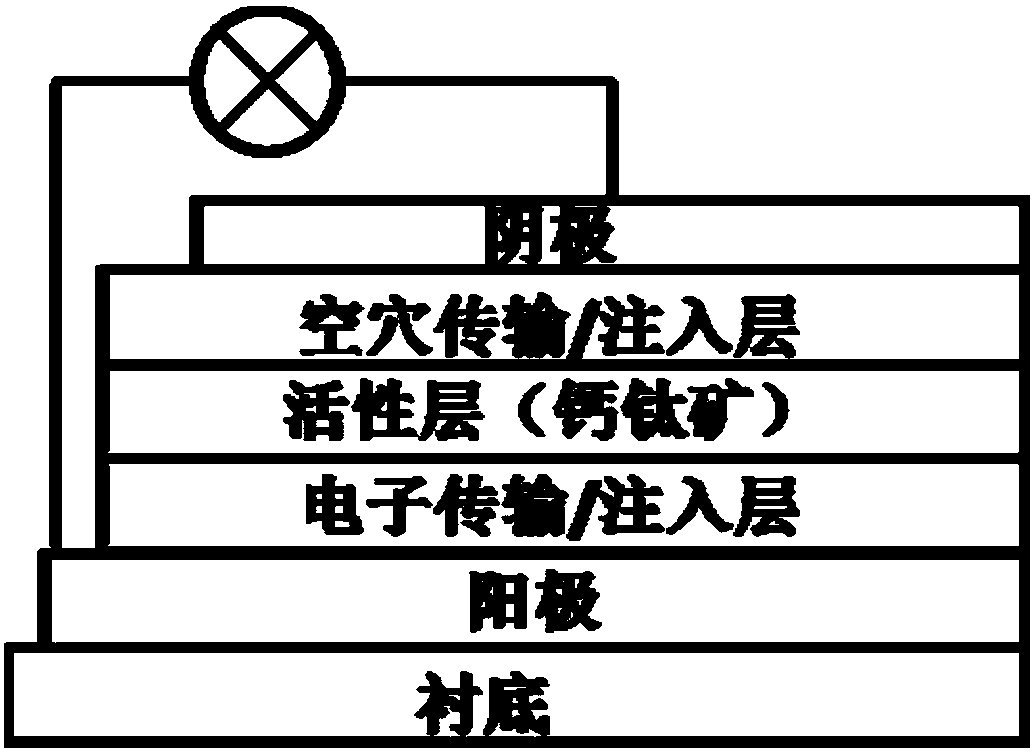
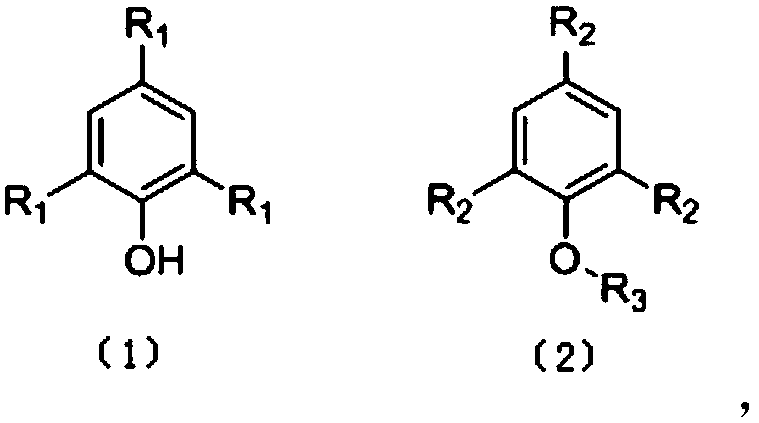
Preparation and application of arylamine derivatives substituted phenol or alkoxybenzene small molecule hole transport materials
A technology of hole transport material and hole transport layer, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, semiconductor/solid-state device manufacturing, etc., can solve the problem of unstudied hole transport performance and unreported arylamine trisubstituted phenol Derivatives and other issues, to achieve good hole transport performance, good electron blocking performance, performance improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The tribromophenol and bromo-n-octane are dissolved in toluene, then sodium hydroxide is added, and the reaction is carried out at 60°C for 5 hours. The reaction product is separated and purified to obtain tribromo-n-octyloxybenzene with a yield of 90%. Dissolve tribromo-octoxybenzene (0.5g) and N-phenylcarbazole-3-boronic acid (2.17g, 7.5mmol) in 100ml of toluene solution, then add 25mL ethanol and 20mL 2M sodium carbonate aqueous solution, nitrogen After venting for 20 minutes, add tetrakistriphenylphosphonium palladium (23mg, 0.02mmol), under nitrogen atmosphere, heat up to 90°C for 12 hours, cool to room temperature, remove the solvent under reduced pressure, separate with silica gel column, and use petroleum ether / two Methyl chloride = 1:1 (v / v) was used as the eluent to obtain the crude product, which was spin-dried to concentrate the solvent, and then washed with petroleum ether three times to obtain a white product with a yield of 30%. 1 H-NMR(DMSO-d 6 ,400MHz)δ8....
Embodiment 2
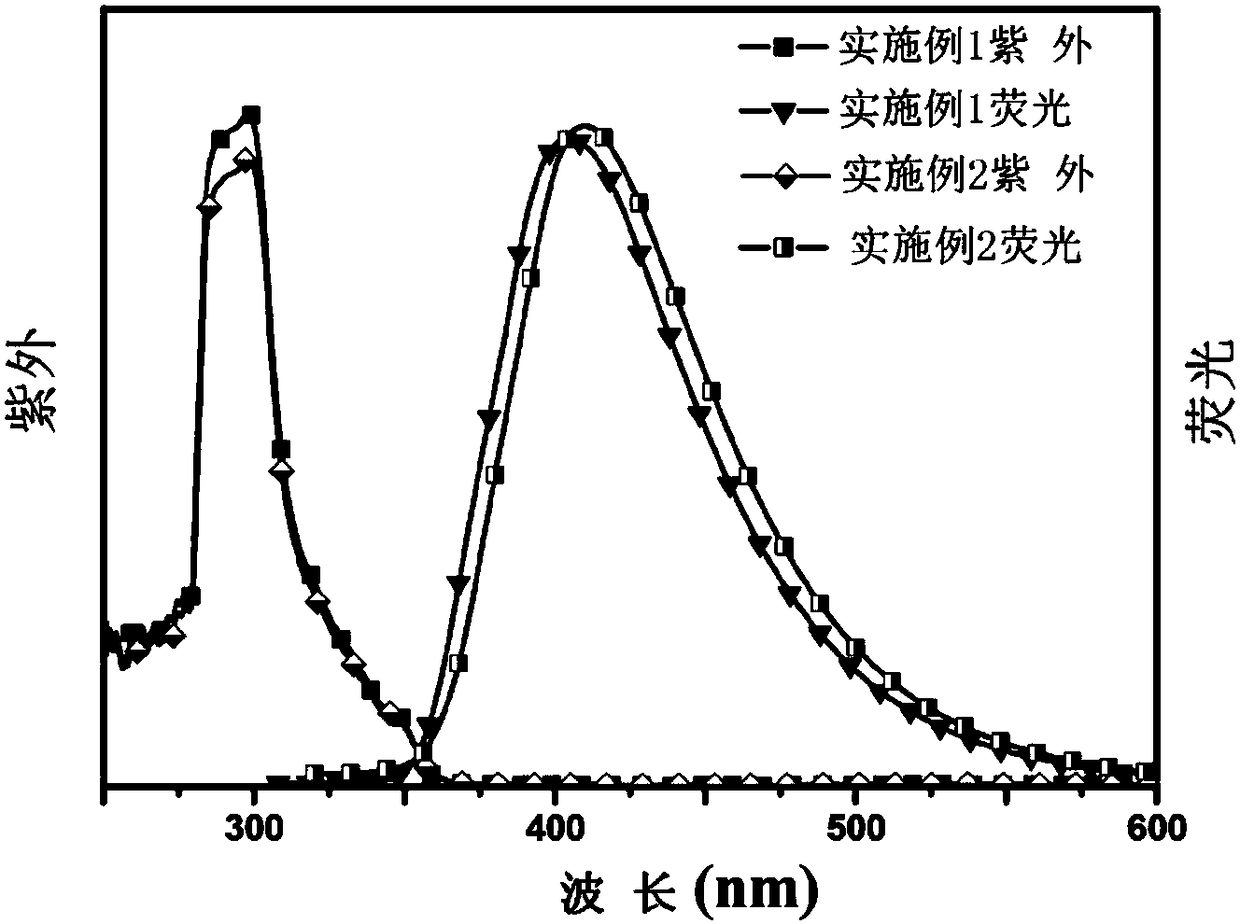
[0040] Dissolve tribromophenol (0.5g) and N-phenylcarbazole-3-boronic acid (2.17g, 7.5mmol) in 100ml of toluene solution, then add 25ml of ethanol and 20ml of 2M sodium carbonate aqueous solution, and vent with nitrogen for 20 minutes , Add palladium tetrakistriphenylphosphorus (23mg, 0.02mmol), under nitrogen atmosphere, heat up to 90℃ and react for 12h, then cool to room temperature, remove the solvent under reduced pressure, separate with silica gel column, with petroleum ether / dichloromethane=1 :1(v / v) is used as the eluent to obtain the crude product, and then washed with petroleum ether 3 times to obtain a pure white product with a yield of 75%. 1 H-NMR(DMSO-d 6 , 400MHz) δ8.72-8.67 (s, 1H); 8.59-8.54 (s, 2H); 8.42-8.31 (m, 4H); 7.90-7.63 (m, 17H); 7.60-7.26 (m, 15H). The solubility of the product in toluene and dimethyl sulfoxide is greater than 20mg / mL, and its ultraviolet absorption and fluorescence intensity curve in toluene solution are as follows figure 2 Shown.
Embodiment 3
[0042] Dissolve tribromophenol and bromo-n-ethane in tetrahydrofuran, then add sodium carbonate aqueous solution and react for 5h at 70°C. The reaction product is separated and purified to obtain tribromoethoxybenzene with a yield of 90%. Dissolve tribromoethoxybenzene (0.5g, 1.5mmol) and 4-(9-carbazolyl)phenylboronic acid (2.60g, 9mmol) in 100ml of dioxane solution, then add 20mL of 2M sodium carbonate aqueous solution After venting with nitrogen for 20 minutes, add tetrakistriphenylphosphonium palladium (23mg, 0.02mmol). Under nitrogen atmosphere, heat up to 110°C and react for 12h, then cool to room temperature, remove the solvent under reduced pressure, and separate with silica gel column. / Dichloromethane=1:1 (v / v) as the eluent to obtain the crude product, and then wash with petroleum ether 3 times to obtain the pure white product, the yield is 75%, and the solubility of the product in toluene is greater than 20mg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com