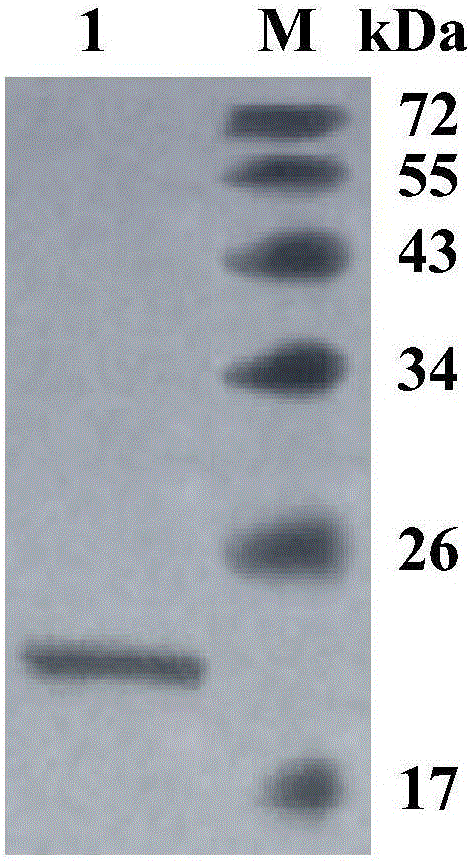
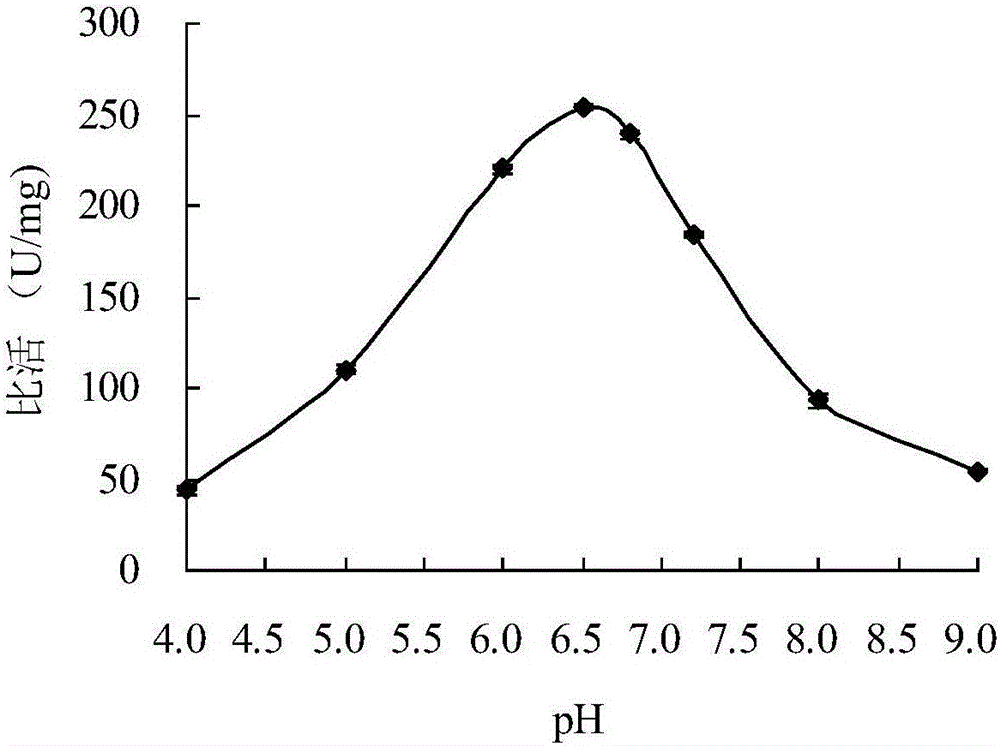
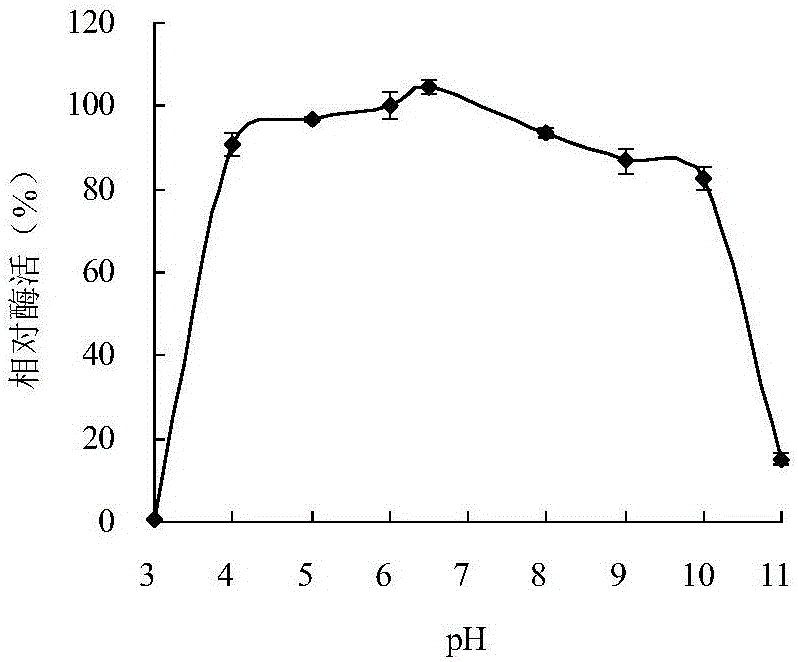
Neutral cold-adaptive xylanase CaXyn11A as well as gene and application thereof
A technology of xylanase and low temperature, applied in the field of genetic engineering, can solve the problem of loss of enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Cladosporium acalyphae SL-16 produces enzyme characteristic
[0048] After culturing Cladosporium acalyphae SL-16 in potato juice medium, the spore suspension was prepared and inoculated in bran liquid medium (Luoetal. % beech xylan was used as the substrate, reacted at pH 6.0 and 30°C for 10 min, and determined to have xylanase activity by the DNS method (Miller1959).
Embodiment 2
[0049] Cloning of Example 2 Cladosporium acalyphae SL-16 Xylanase Encoding Gene Caxyn11A
[0050] Extraction of Cladosporium acalyphae SL-16 genomic DNA:
[0051] Filter the mycelium cultured in liquid for 3 days with sterile filter paper, put it into a mortar, add 2mL of extract, grind for 5min, then put the grinding solution in a 50mL centrifuge tube, lyse in a water bath at 65°C for 20min, and mix every 10min. Homogenize once and centrifuge at 10,000 rpm for 5 min at 4°C. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 5 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuum, dissolved by adding an appropriate amount of TE, and stored at -20°C for later use.
[0052] Design and synthesis of specific primers Caxyn11A-F / Caxyn11A-F:
[0...
Embodiment 3
[0056] RT-PCR Analysis of Example 3 Cladosporium acalyphae SL-16 Xylanase Gene
[0057] Extract the total RNA of Cladosporium acalyphae SL-16, use reverse transcriptase to obtain a strand of cDNA, then amplify the single-stranded cDNA with primers Caxyn11A-E-F / Caxyn11A-E-F, obtain the cDNA sequence of mature xylanase, and amplify The recovered product was sent to Sanbo Biotechnology Co., Ltd. for sequencing.
[0058] Caxyn11A-E-F: 5'-GGGCATATGATGGTCGGAATCAAGTCCCTTCTCCTCGC-3';
[0059] Caxyn11A-E-R: 5'-GGGGAATTCCTAGTGGTGGTGGTGGTGGTGAGACGTCTG
[0060] AACGTAGATGTCAGCG-3'.
[0061] By comparing the genome sequence and cDNA sequence of xylanase, it was found that the gene had 2 introns, the cDNA was 696 bp long, encoded 231 amino acids and a stop codon, and the N-terminal 19 amino acids were its signal peptide sequence. The measured partial nucleotide sequence of the mature protein of the gene Caxyn11A was homologously compared with the xylanase gene sequence on GenBank, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com