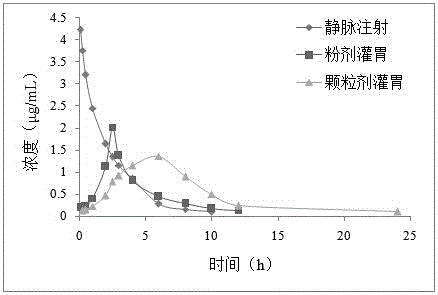
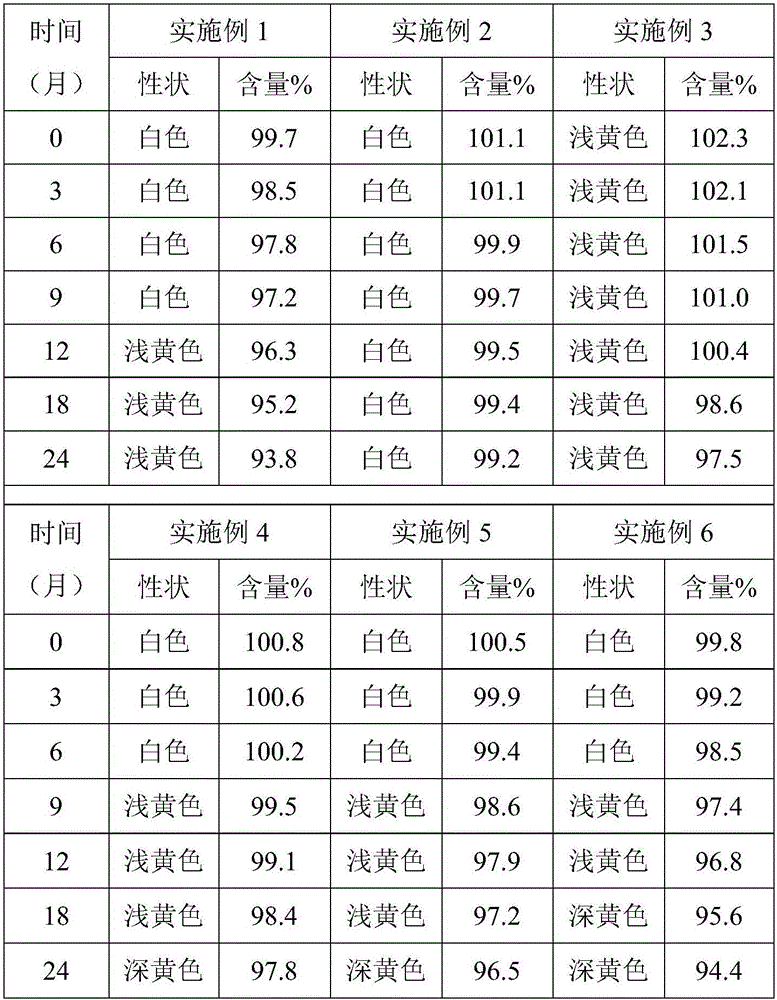
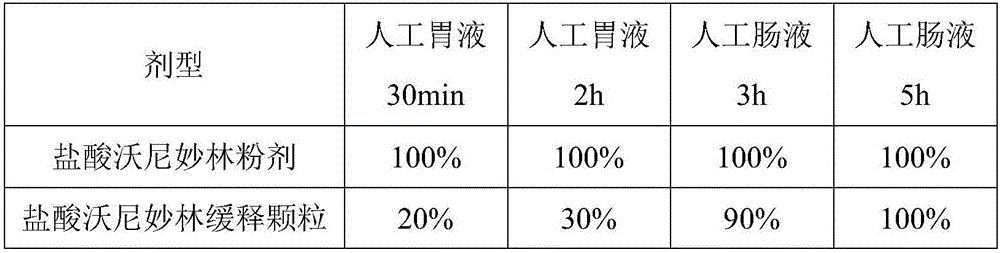
Valnemulin hydrochloride sustained-release granules and preparation method and application thereof
A technology of warnemulin hydrochloride and sustained-release granules, which can be applied in the direction of pharmaceutical formulations, block delivery, and active ingredients of esters, etc., and can solve the problems of cumbersome clinical application of injections, difficulty in mixing uniform feed, gastric mucosal irritation, etc. , to achieve the effect of solving the problem of drug palatability, prolonging the maintenance time of effective blood drug concentration, and stabilizing blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of Warnemulin Hydrochloride Sustained Release Granules
[0044] The preparation steps are as follows:
[0045] S1. Weigh each carrier auxiliary material according to the following formula ratio (based on the total mass as 100g):
[0046] Warnemulin hydrochloride 20%;
[0047] Stearyl alcohol 75%;
[0048] Beeswax 5%;
[0049] Heat to melt at 80°C and mix well;
[0050] S2. adding warnemulin hydrochloride to the carrier auxiliary material obtained in S1, and stirring evenly to obtain a mixed material;
[0051] S3. Cool the mixed material obtained in S2 to 60-80°C, and perform the stage of fluidized bed spray granulation and spheroidization, the temperature of the fluidized air in the granulation and spheroidization stage is 25°C; the fluidized bed spray droplets The line speed is 10-160 m / s;
[0052] S4. Cool, sieve, and collect to obtain 20% warnemulin hydrochloride sustained-release granules.
Embodiment 2
[0053] Example 2 Preparation of Warnemulin Hydrochloride Sustained Release Granules
[0054] The preparation steps are as follows:
[0055] S1. Weigh each carrier auxiliary material according to the following formula ratio (based on the total mass as 100g):
[0056] Warnemulin hydrochloride 10%;
[0057] Glyceryl monostearate 80%;
[0058] Beeswax 10%;
[0059] Heat to melt at 70°C and mix well;
[0060] S2. adding warnemulin hydrochloride to the carrier auxiliary material obtained in S1, and stirring evenly to obtain a mixed material;
[0061] S3. Cool the mixed material obtained in S2 to 60-80°C, and carry out the stage of fluidized bed spray granulation and spheroidization, the temperature of the fluidized air in the granulation and spheroidization stage is 20°C; The line speed is 40-80 m / s;
[0062] S4. Cool, sieve, and collect to obtain 10% warnemulin hydrochloride sustained-release granule preparation.
Embodiment 3
[0063] Example 3 Preparation of Warnemulin Hydrochloride Sustained Release Granules
[0064] The preparation steps are as follows:
[0065] S1. Weigh each carrier auxiliary material according to the following formula ratio (based on the total mass as 100g):
[0066] Warnemulin hydrochloride 25%;
[0067] Animal fat 70%;
[0068] Carnauba wax 5%;
[0069] Heat to melt at 95°C and mix well;
[0070] S2. adding warnemulin hydrochloride to the carrier auxiliary material obtained in S1, and stirring evenly to obtain a mixed material;
[0071] S3. Cool the mixed material obtained in S2 to 60-80°C, and carry out the stage of fluidized bed spray granulation and spheroidization, the fluidized air temperature in the granulation and spheroidization stage is 30°C; the fluidized bed spray droplets The line speed is 40-80 m / s;
[0072] S4. Cool, sieve, and collect to obtain 25% warnemulin hydrochloride sustained-release granule preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com