Preparation method of polymeric ferric sulfate
A technology for polymerizing ferric sulfate and ferrous sulfate, which is applied in the direction of ferric sulfate, can solve the problems of high energy costs, etc., and achieve the effects of short production cycle, high stability and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
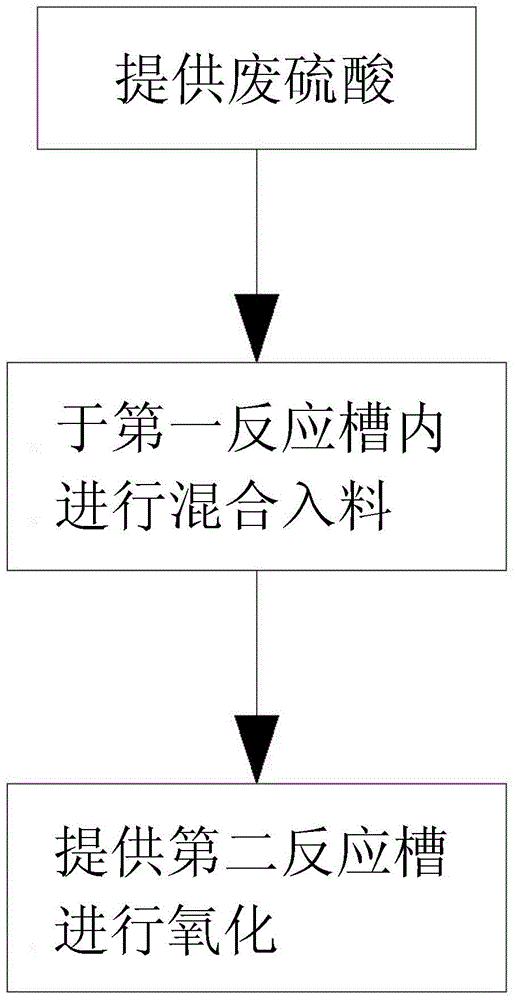
[0023] Such as figure 1 As shown, the preparation method of polymeric ferric sulfate of the present invention basically includes the following steps in sequence.
[0024] Step a: Provide waste sulfuric acid, such as waste sulfuric acid produced in the semiconductor industry, photoelectric industry or other industries. The content of sulfuric acid in the waste sulfuric acid is generally 20-80%.
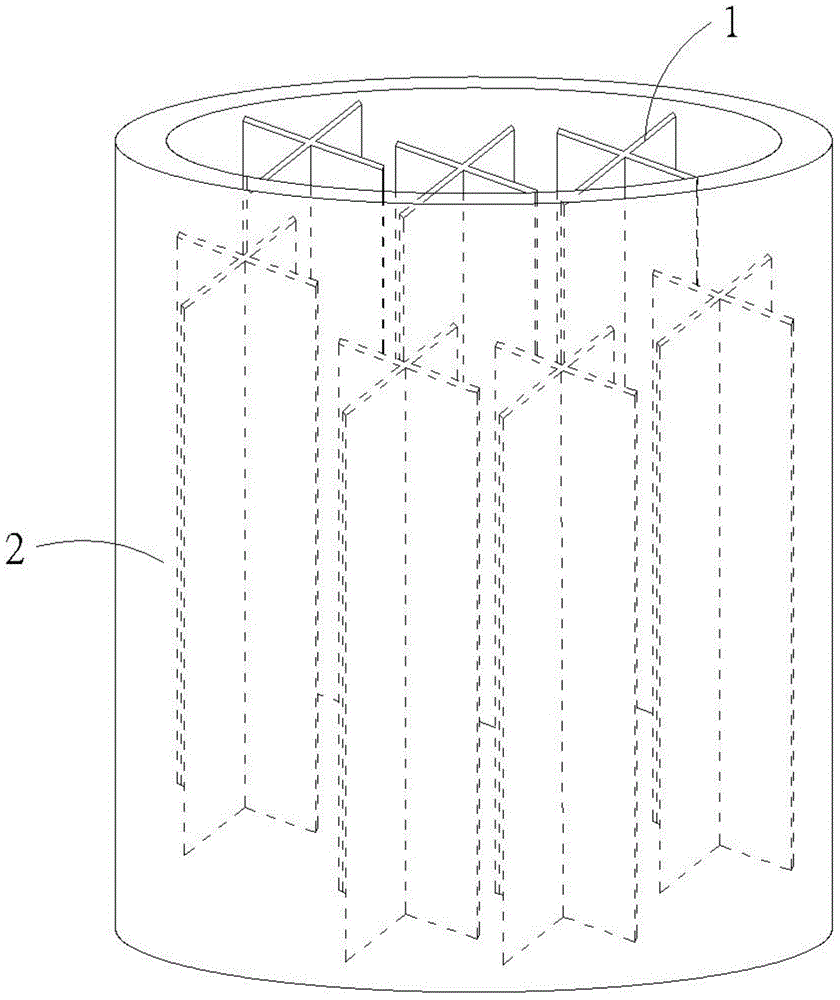
[0025] Step b: Perform mixing and feeding, and place at least one iron piece 1, the above-mentioned waste sulfuric acid and water in a first reaction tank 2 in sequence, please refer to figure 2 As shown, the iron flakes 1 can be multiple in a radial shape to increase the reaction area, and the volume ratio of waste sulfuric acid to water is 1:1.5 to 1:3; for example, the volume ratio of waste sulfuric acid to water is added 1:2 is better, the temperature in the first reaction tank 2 is controlled at 30-50°C; and the pH in the first reaction tank 2 is controlled at 1-3, and the reaction tim...
Embodiment 1
[0030] Take an appropriate amount of iron flakes, put it into the reaction tank, and add about 500L of waste sulfuric acid, and then slowly add about 1500L of tap water to react. The reaction time is about 15 minutes, the pH is about 1±0.5, and the reaction temperature is about 50°C. When the content of ferrous iron in the liquid in the reaction tank is ≧3%, it is semi-finished ferrous sulfate; after filtering the semi-finished ferrous sulfate, take 2000L of ferrous sulfate, 20L tap water and 60L sulfuric acid into the second reaction tank , And introduce about 60L of hydrogen peroxide from the bottom of the tank to react. The reaction time is 10 minutes, and the reaction temperature is about 30°C. When the weight percentage of liquid divalent iron converted into trivalent iron content in the reaction tank is greater than or equal to 3%, that is It is the finished polymer ferric sulfate (PFS).
Embodiment 2
[0032] Take a proper amount of iron flakes, put it into the reaction tank, add about 500L of waste sulfuric acid, and then slowly add about 750L of tap water to react. The reaction time is about 10 minutes, the pH is about 2±0.5, and the reaction temperature is about 55°C. When the content of ferrous iron in the liquid in the reaction tank is ≧3%, it is semi-finished ferrous sulfate; after filtering this semi-finished ferrous sulfate, take 2000L of ferrous sulfate, 20L tap water and 60L sulfuric acid into the second reaction tank , And introduce about 60L of hydrogen peroxide from the bottom of the tank to react. The reaction time is 10 minutes, and the reaction temperature is about 35°C. When the weight percentage of liquid divalent iron converted into trivalent iron content in the reaction tank is greater than or equal to 3%, that is It is the finished polymer ferric sulfate (PFS).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com