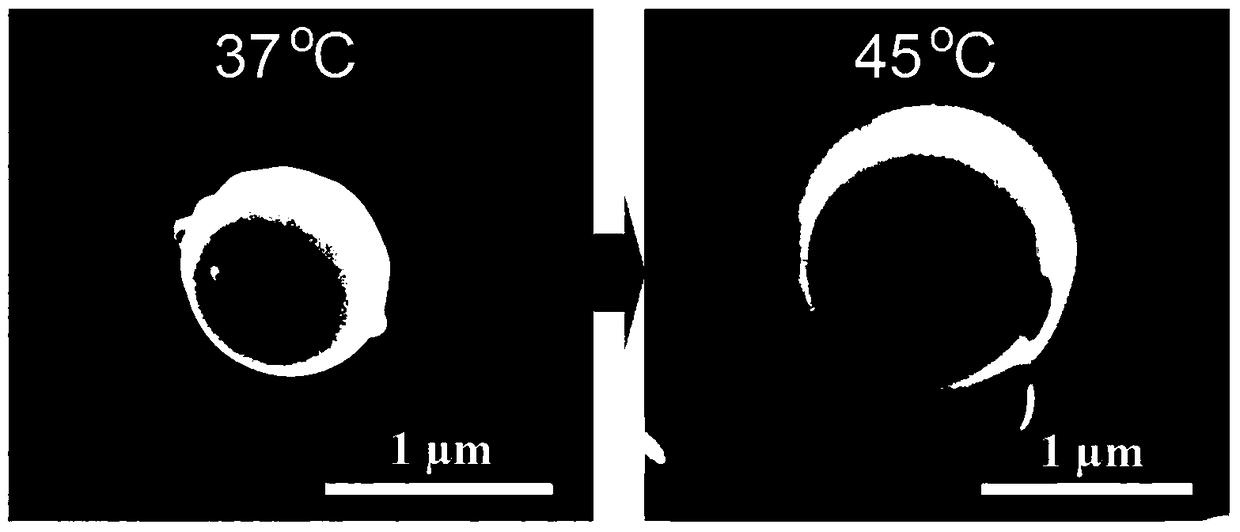
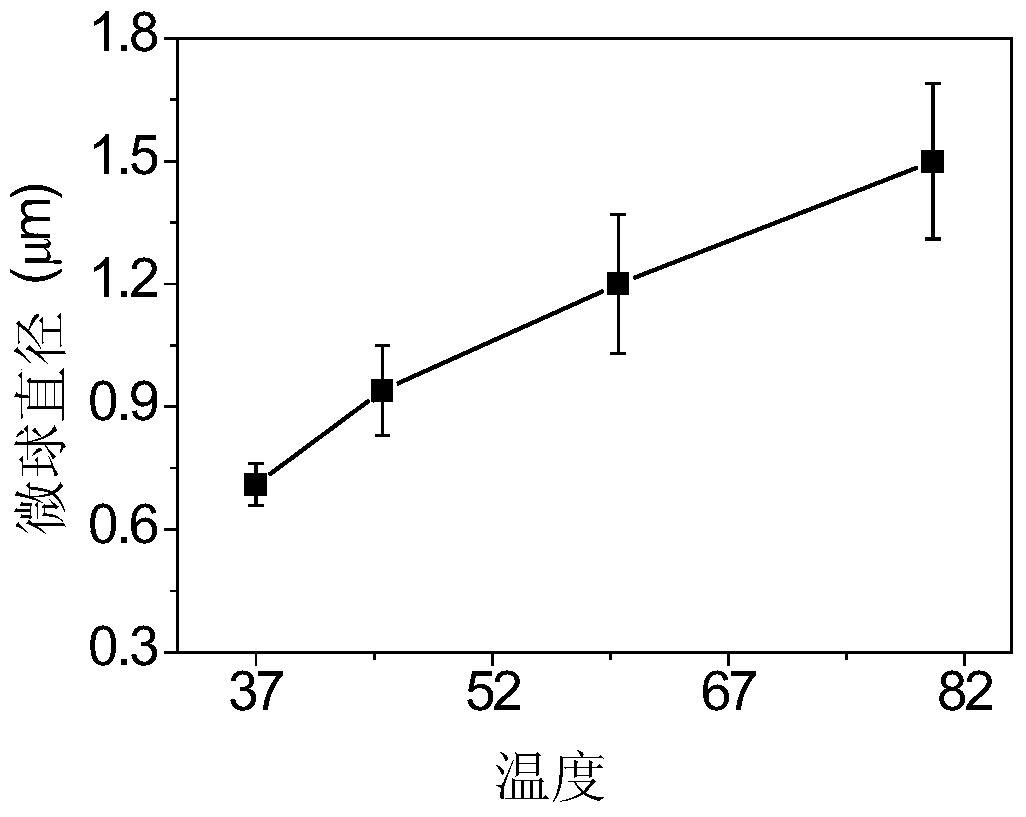
A kind of preparation method of temperature-controlled drug release polymer microsphere material
A polymer and microsphere technology, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The ball loses the slow release ability and other problems, so as to achieve the effect of retaining natural activity, protecting biological activity and fast curing speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the temperature-controlled drug release polymer microsphere material of the present invention specifically comprises the following steps:
[0032] Step 1, under room temperature, 0.5 gram of chitosan is dissolved in 40 milliliters of concentration and is the lactic acid solution of 5% (v / v), stirs 3 hours to form homogeneous solution, then adds the succinic anhydride of 1~4 times of chitosan quality, Stir at room temperature for 12 to 24 hours to react, dialyze and freeze-dry the reaction solution to obtain water-soluble succinyl chitosan;
[0033] Step 2. Dissolve 0.5 g of succinyl chitosan in 40 ml of phosphate buffer solution at room temperature, add 100 to 500 microliters of furfurylamine-2-methylfurylamine dropwise into the solution, and then add 1 / 5 Carbodiimide with ~1 / 2 times the mass of succinyl chitosan was stirred at room temperature for 12 to 24 hours to react, dialyzed and freeze-dried to obtain furanated chitosan;
[0034] Step 3...
Embodiment 1
[0043] (1) Add 0.5 gram of chitosan in 40 milliliters of 5% (v / v) lactic acid solution, stir at room temperature for 3 hours to form a uniform solution, then add 2 grams of succinic anhydride, stir at room temperature for 24 hours, then react The solution was dialyzed and freeze-dried to obtain succinyl chitosan;
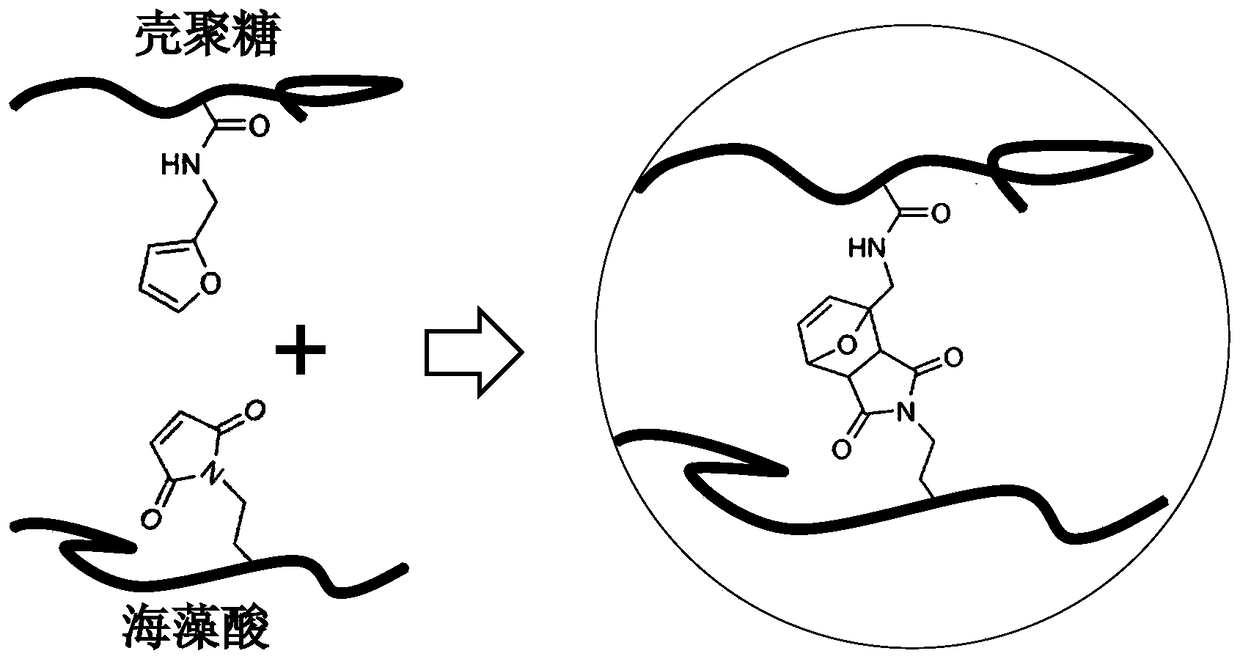
[0044] (2) Dissolve 0.5 g of succinyl chitosan in 40 ml of buffer solution at room temperature, then add 500 microliters of furfurylamine-2-methylfurylamine dropwise, add 0.25 g of carbodiimide and stir at room temperature for 24 hours , dialyzed and freeze-dried to obtain furanated chitosan (the structure diagram is shown in figure 1 );
[0045] (3) Dissolve 1.0 g of sodium alginate in 100 ml of water at room temperature, then add 5 ml of sodium periodate solution with a concentration of 0.5 M dropwise, stir for 2 hours in the dark, dialyze and freeze-dry to obtain aldylated alginic acid sodium;
[0046] (4) Dissolve 1.0 g of alginate sodium alginate in 100 ml o...
Embodiment 2
[0054] (1) Add 0.5 gram of chitosan in 40 milliliters of 5% (v / v) lactic acid solution, stir at room temperature for 3 hours, form a uniform solution, then add 1.5 gram of succinic anhydride, stir at room temperature for 20 hours, then react The solution was dialyzed and freeze-dried to obtain succinyl chitosan;
[0055] (2) Dissolve 0.5 g of succinyl chitosan in 40 ml of buffer solution at room temperature, then add 400 microliters of furfurylamine-2-methylfurylamine dropwise, add 0.2 g of carbodiimide and stir at room temperature for 20 hours , dialysis and freeze-drying to obtain furanated chitosan;
[0056] (3) Dissolve 1.0 g of sodium alginate in 100 ml of water at room temperature, then add dropwise 4.5 ml of sodium periodate solution with a concentration of 0.5 M, stir for 3 hours in the dark, dialyze and freeze-dry to obtain alginic acid alginate sodium;
[0057] (4) Dissolve 1.0 gram of alginate sodium alginate in 100 milliliters of water at room temperature, then a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com