Method for synthesizing (R)-2-hydroxy acid by biological enzyme method
A bio-enzyme method and hydroxy acid technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of unavailable commercial production, high equipment costs, and increased production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Construction of recombinant strain E.coliBL21(DE3) / pET28b-HADH1 / pCDFDuet-KAR-GDH
[0061] (1) Design primer pair P1-F: GGAATTCCATATGATGATCATTTCCGCTTCCACC, P1-R: CCCAAGCTTTCAGGCGCCCAGTTCGCGGACCA. The genomic DNA in PseudomonasaeruginosaCCTCCM2011394 was used as a PCR template for PCR amplification. The PCR reaction process was as follows: 95°C for 5min, 95°C for 1min, 57°C for 1min, 72°C for 1min (30cycles); 72°C for 10min, 4°C forever.
[0062] The reaction system is as follows:
[0063]
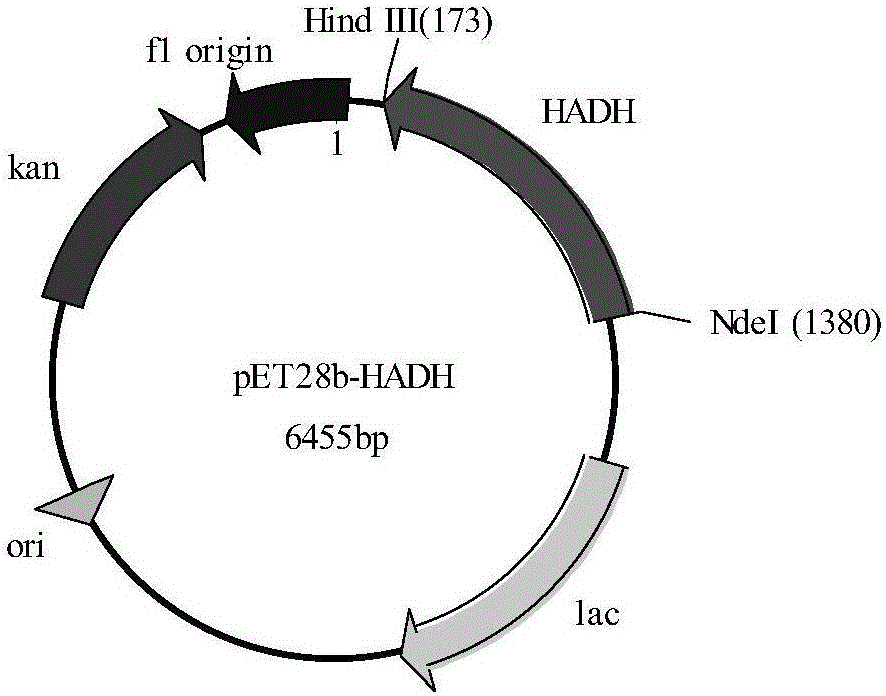
[0064] The amplified product is the gene sequence of 2-hydroxyacid dehydrogenase 2-HADH, which is denoted as HADH1 (the nucleotide sequence is shown in SEQ ID NO.1, and the amino acid sequence is shown in SEQ ID NO.2). The PCR product was purified by agarose gel electrophoresis, and the target band was recovered using an agarose gel DNA recovery kit. After double digestion, it was ligated with the expression vector pET28b and transformed into E.coliBL21(DE3) to construct the E.co...
Embodiment 2
[0076] Construction of recombinant strain E.coliBL21(DE3) / pET28b-HADH2 / pCDFDuet-KAR-GDH
[0077] Synthesize the 2-hydroxyacid dehydrogenase 2-HADH gene sequence in Burkholderiaxenovorans LB400 (ABE35802.1), denoted as HADH2 (nucleotide sequence is shown in SEQIDNO.3, amino acid sequence is shown in SEQIDNO.4), connected to pET28b And it was transferred into E.coliBL21(DE3) to construct E.collBL21(DE3) / pET28b-HADH2 strain.
[0078] The construction of the recombinant plasmid pCDFDuet-KAR-GDH is the same as in Example 1, refer to figure 2 .
[0079] The three-enzyme expression system was constructed by the method of double plasmids in one bacterium, and the plasmids pET28b-HADH2 and pCDFDuet-KAR-GDH were mixed at a ratio of 1:1, and then transferred into E.coliBL21(DE3) by conventional heat shock method or electric shock method In the competent state, spread it on a double-antibody plate containing streptomycin and kanamycin, and select the E.coliBL21(DE3) / pET28b-HADH2 / pCDFDu...
Embodiment 3
[0081] Construction of recombinant strain E.coliBL21(DE3) / pET28b-HADH3 / pCDFDuet-KAR-GDH
[0082] The 2-hydroxyacid dehydrogenase 2-HADH gene sequence in the synthetic Pseudomonasputida is recorded as HADH3 (the nucleotide sequence is shown in SEQ ID NO.5, and the amino acid sequence is shown in SEQ ID NO.6), connected to pET28b and transferred to E .coliBL21(DE3), construct E.collBL21(DE3) / pET28b-HADH3 strain.
[0083] The construction of the recombinant plasmid pCDFDuet-KAR-GDH is the same as in Example 1, refer to figure 2 .
[0084] Construct a three-enzyme expression system by the method of double plasmids in one bacterium, mix the plasmids pET28b-HADH3 and pCDFDuet-KAR-GDH at a ratio of 1:1, and transfer them into E.coliBL21(DE3) by conventional heat shock method or electric shock method In the competent state, spread it on a double-antibody plate containing streptomycin and kanamycin, and select the E.coliBL21(DE3) / pET28b-HADH3 / pCDFDuet-KAR-GDH strain, and the operati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com