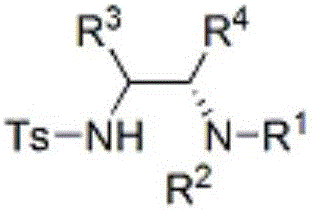
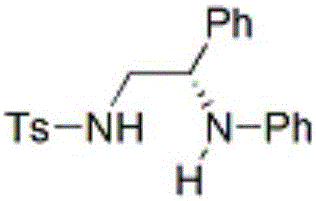
Chiral vicinal diamine compound and method for preparing same
A technology for ortho-diamine compounds, applied in the field of chiral ortho-diamine compounds and their preparation, to achieve the effects of simple reaction raw materials, rich product structure types, and easy-to-obtain reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under an argon atmosphere, silver hexafluoroantimonate (0.0025 mmol), (S)-DTBM-SEGPHOS (0.00375 mmol) and dry m-xylene (0.25 mL) were successively added to a dry Schlenk tube, and stirred at room temperature for 30 minutes 2-Phenyl-N-p-toluenesulfonyl aziridine (0.22mmol) and aniline (0.1mmol dissolved in 0.25mL dry m-xylene) were added in sequence, and the reaction was stirred at 15°C for 10 hours (monitored by TLC). After completion of the reaction, add saturated aqueous sodium bicarbonate solution (5mL) to stop the reaction, extract with ethyl acetate (5mL×3), dry over anhydrous magnesium sulfate, and remove the solvent under reduced pressure to obtain the crude product. The mixed solvent of 1 (containing 0.5v% triethylamine) is used as a developing agent, and the product is separated by 300-400 mesh silica gel column chromatography, and its enantiomeric ratio (e.r. value) is determined by the HPLC method of a chiral stationary phase, and the yield : 35.5 mg, yield: ...
Embodiment 2
[0033]Under an argon atmosphere, silver hexafluoroantimonate (0.0025 mmol), (S)-DTBM-SEGPHOS (0.00375 mmol) and dry m-xylene (0.25 mL) were successively added to a dry Schlenk tube, and stirred at room temperature for 30 minutes Add 2-phenyl-N-p-toluenesulfonyl aziridine (0.22mmol) and 4-methoxyaniline (0.1mmol dissolved in 0.25mL dry m-xylene) in sequence, and then stir the reaction at 15°C for 10 hours (TLC monitoring). After the reaction was completed, saturated aqueous sodium bicarbonate solution (5 mL) was added to terminate the reaction, extracted with ethyl acetate (5 mL×3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product. The mixed solvent of 1 (containing 0.5v% triethylamine) is used as a developing agent, and the product is separated by 300-400 mesh silica gel column chromatography, and its enantiomeric ratio (e.r. value) is determined by the HPLC method of a chiral stationary phase, and the yield ...
Embodiment 3
[0037] The operation of reaction is with embodiment 2, but solvent used is different, and concrete operation is as follows:
[0038] Under an argon atmosphere, silver hexafluoroantimonate (0.0025 mmol), (S)-DTBM-SEGPHOS (0.00375 mmol) and dry dichloromethane (0.25 mL) were successively added to a dry Schlenk tube, and stirred at room temperature for 30 minutes Add 2-phenyl-N-p-toluenesulfonyl aziridine (0.22mmol) and 4-methoxyaniline (0.1mmol dissolved in 0.25mL dry dichloromethane) in sequence, and then stir the reaction at 15°C for 12 hours (TLC monitoring). After the reaction was completed, saturated aqueous sodium bicarbonate solution (5 mL) was added to terminate the reaction, extracted with ethyl acetate (5 mL×3), dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure to obtain a crude product. The mixed solvent of 1 (containing 0.5v% triethylamine) is used as a developing agent, and the product is separated by 300-400 mesh silica gel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com