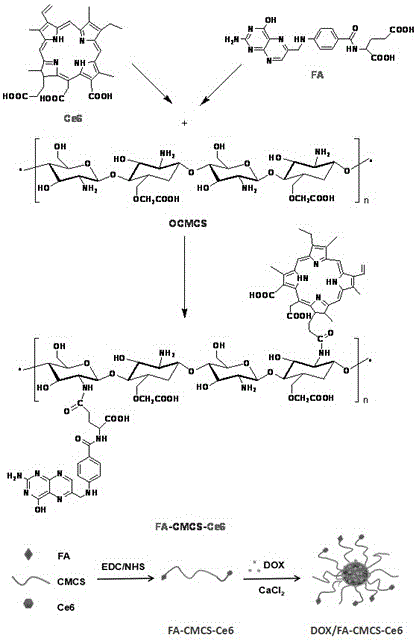
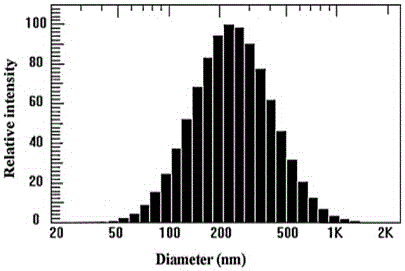
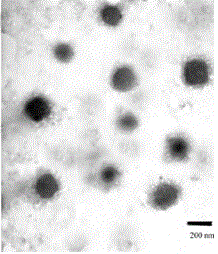
Preparation method and application of tumor-targeted nanometer drug delivery system for cooperative chemotherapy and photodynamic therapy
A photodynamic therapy and drug delivery system technology, applied in the field of biomedicine, can solve the problems of limited application of photodynamic therapy, limited phototoxicity, poor water solubility, etc., and achieve orderly release, high drug loading rate, good slowness The effect of controlled release capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of blank FA-CMCS-Ce6 conjugates
[0026] a. Dissolve Ce6 in 0.1% (v / v) dimethylsulfoxide (DMSO) aqueous solution, add 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), reacted at room temperature in the dark for 0.5 hours, then added the activated Ce6-NHS to the CMCS aqueous solution, adjusted the pH to 4.5~5.0, stirred overnight at room temperature in the dark, and obtained Product CMCS-Ce6. The molar ratio of Ce6 / EDC / NHS added therein is 0.5:1:1.1, and the molar ratio of Ce6 / CMCS is 20:1. The product CMCS-Ce6 was placed in a centrifuge, centrifuged at 14,000 rpm for 5 minutes to remove aggregates, and then filtered through an ultrafiltration membrane (molecular weight cut-off 10kDa) to remove free Ce6 active esters to obtain purified CMCS-Ce6.
[0027] b. Dissolve FA in 0.1% (v / v) dimethyl sulfoxide (DMSO) aqueous solution, add dicyclohexylcarbodiimide (DCC) and NHS, react in the dark for 1 hour ...
Embodiment 2
[0028] Example 2: Preparation of a tumor-targeted nano drug delivery system loaded with doxorubicin
[0029] The ion cross-linking method was used to prepare the drug-loaded nanoparticles with CaCl2 as the cross-linking agent. Dissolve the lyophilized FA-CMCS-Ce6 in water, adjust the pH to 7.2, slowly add a certain amount of DOX·HCl aqueous solution into the FA-CMCS-Ce6 solution under stirring, dissolve with 40W ultrasonic, and then add the CaCl2 solution with Drop into the above FA-CMCS-Ce6 solution at a speed of 3 s / drop, and continue to stir for 30 minutes to obtain a DOX / FA-CMCS-Ce6 nanoparticle suspension. Centrifuge the suspension at 16,000 rpm for 30 minutes, collect The precipitate was freeze-dried to obtain DOX / FA-CMCS-Ce6 nanoparticles. The mass ratio of the added crosslinking agent CaCl2 to FA-CMCS-Ce6 is 1:5, and the mass ratio of DOX dosage to FA-CMCS-Ce6 is 1:10.
[0030] figure 1 It is a schematic diagram of synthesis and preparation of DOX / FA-CMCS-Ce6 nanopa...
Embodiment 3
[0032] Example 3: In vitro drug release of DOX / FA-CMCS-Ce6 nanoparticles
[0033] Add DOX solution and DOX / FA-CMCS-Ce6 nanoparticles (both at a concentration of 1 mg / ml of DOX) to phosphate buffer at pH 7.4 (50 μmol / L) and transfer to a dialysis tube (molecular weight cut-off 1 kDa ), place the dialysis tube in 10 ml of phosphate buffer, and place it in a constant temperature shaker (50 rpm) at 37°C to measure the release. At the designated time point, the solution outside the dialysis tube was taken, and an equal volume of fresh buffer solution was added at the same time, and the content of doxorubicin was determined by ultraviolet spectrophotometry.
[0034] Figure 4 It is the result of in vitro release of doxorubicin. When the pH of the release medium is 5.5 and 7.4, the release has a burst release phenomenon, which is mainly caused by the drug adsorbed on the outer layer of the nanoparticles. After 2 hours, the release of the carrier drug enters the slow and controlled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com