Method for preparing iron phthalocyanine-graphene porous composite material
A porous composite material, graphene technology, applied in the field of electrochemical catalyst preparation, can solve problems such as unsuitable practical application, low solubility, and complicated operation, and achieve the effects of reducing complexity and cost, good porous structure, and simplifying experimental steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
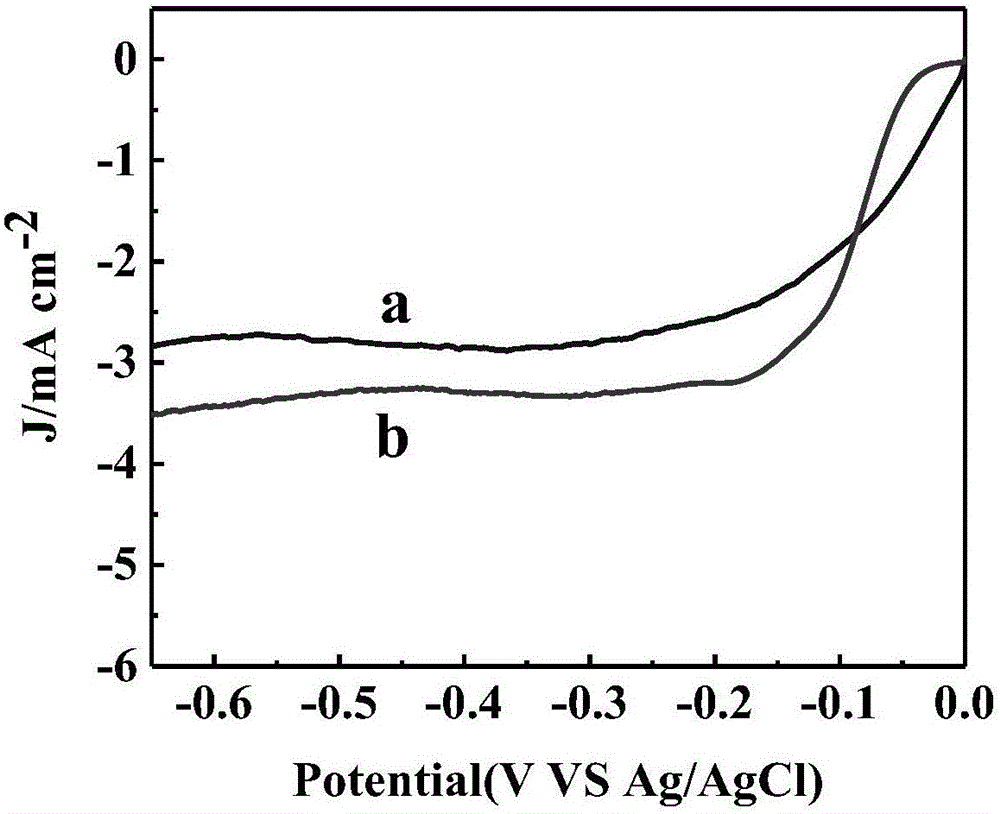
[0025] Weigh 20 mg of graphene oxide into a beaker, add 5 mL of water and ultrasonically disperse for 30 min to obtain a uniform dispersion. Then put the beaker into liquid nitrogen, and after the graphene oxide dispersion is completely frozen to a solid state, put it into a freeze dryer (the freezing temperature has reached -50°C), turn on the vacuum mode, and make the vacuum freeze dryer Operate in this mode for 40 hours, and the graphene oxide porous composite material will be obtained after the water in the sample is completely sublimated. Then, the graphene oxide porous composite was calcined in a nitrogen atmosphere at 700 °C for 1.5 h at a heating rate of 3 °C / min. After the calcination, the sample is taken out to obtain a graphene porous material. Use an electronic analytical balance to weigh 0.3 mg of iron phthalocyanine powder into a small glass bottle, add 3 mL of absolute ethanol, and ultrasonically disperse for 30 minutes to obtain a uniform concentration of 0.1 ...
Embodiment 2
[0027] Weigh 30 mg of graphene oxide into a beaker, add 5 mL of water and ultrasonically disperse for 30 min to obtain a uniform dispersion. Then put the beaker into liquid nitrogen, and after the graphene oxide dispersion is completely frozen to a solid state, put it into a freeze dryer (the freezing temperature has reached -50°C), turn on the vacuum mode, and make the vacuum freeze dryer Operate in this mode for 45 hours, and the graphene oxide porous composite material will be obtained after the water in the sample is completely sublimated. Then, the graphene oxide porous composite was calcined in a nitrogen atmosphere at 750 °C for 2 h at a heating rate of 3 °C / min. After the calcination, the sample is taken out to obtain a graphene porous material. Use an electronic analytical balance to weigh 0.6mg of iron phthalocyanine powder into a small glass bottle, add 3mL of absolute ethanol, and ultrasonically disperse for 30min to obtain a uniform concentration of 0.2mgmL -1 s...
Embodiment 3
[0030]Weigh 35 mg of graphene oxide into a beaker, add 5 mL of water and ultrasonically disperse for 30 min to obtain a uniform dispersion. Then put the beaker into liquid nitrogen, and after the graphene oxide dispersion is completely frozen to a solid state, put it into a freeze dryer (the freezing temperature has reached -50°C), turn on the vacuum mode, and make the vacuum freeze dryer Operate in this mode for 48 hours, and the graphene oxide porous composite material will be obtained after the water in the sample is completely sublimated. Then, the graphene oxide porous composite was calcined at 800 °C for 3 h under the protection of nitrogen atmosphere, and the heating rate was 4 °C / min. After the calcination, the sample is taken out to obtain a graphene porous material. Weigh 1.2 mg of iron phthalocyanine powder into a small glass bottle with an electronic analytical balance, add 3 mL of absolute ethanol, and ultrasonically disperse for 40 minutes to obtain a uniform co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peak current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com