Strains, application of strains, vaccine and preparation method of vaccine
A strain and vaccine technology, applied in the biological field, can solve the problem of low cross-protection rate, reduce mink stress response, have good market application prospects, and prevent mink hemorrhagic pneumonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of Mink Parvovirus Enteritis and Hemorrhagic Pneumonia Dual Inactivated Vaccine
[0057] 1. Standards for vaccine strains:
[0058] 1.1 Pseudomonas aeruginosa strain standard
[0059] 1.1.1 Morphological and biochemical characteristics The bacteria are Gram-negative bacilli. The biochemical characteristics should conform to the characteristics of the bacteria in bacterial taxonomy.
[0060] 1.1.2 Culture characteristics Inoculate on a PYG agar plate and culture at 36-37°C for 18-24 hours. Visual observation shows that the colonies are flat and moist, with irregular edges, and produce green or brown pigments that diffuse in the medium; inoculate with PYG liquid In the culture medium, culture at 36-37°C for 18-24 hours, the liquid will be evenly turbid and produce green or brown pigment.
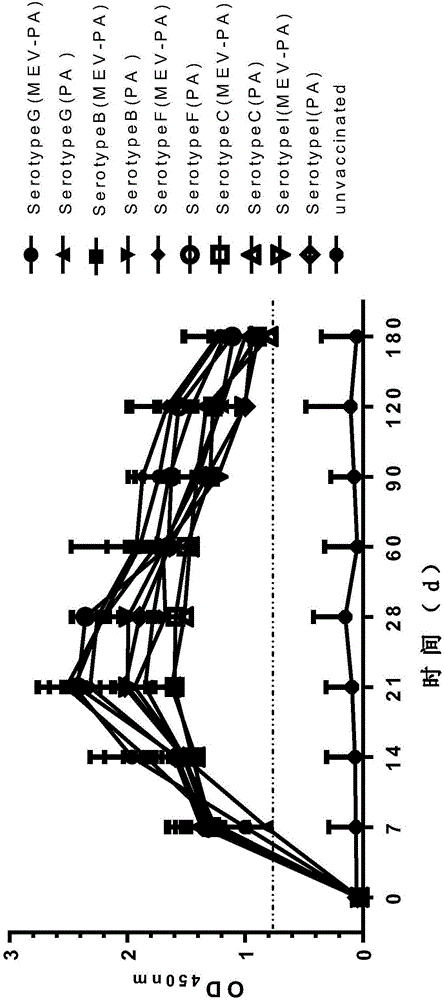
[0061] 1.1.3 Serological characteristics Use Pseudomonas aeruginosa type serotype, DL15 strain should be G type, JL08 strain should be B type, WD01 strain shoul...
Embodiment 2
[0145] Embodiment 2 safety test
[0146] Table 2 Safety test inspection content
[0147]
[0148] 1.1 Safety test
[0149] Three batches of vaccines 201501, 201502, and 201503 were inoculated in a single dose, repeatedly inoculated with a single dose, and overdose inoculated minks of different ages for safety evaluation. A single dose was inoculated with young minks aged 60 to 70 days, 1ml / mink; single-dose repeated inoculation Young minks aged 60-70 days, with an interval of 15 days between two vaccinations, each inoculation of 1ml / mink; over-dose inoculation of young minks aged 60-70 days and adult minks aged 3-6 months, 3ml / mink, injected in 3 points . In each group, 5 minks were inoculated, and the inner muscles of the hind limbs were inoculated, and a non-immunized control group was established. Continuously observe for 15 days after inoculation, observe systemic clinical symptoms and local changes of inoculation, and record body temperature changes every morning for ...
Embodiment 3
[0155] Efficacy Evaluation of Embodiment 3MEV-PA Dual Inactivated Vaccine
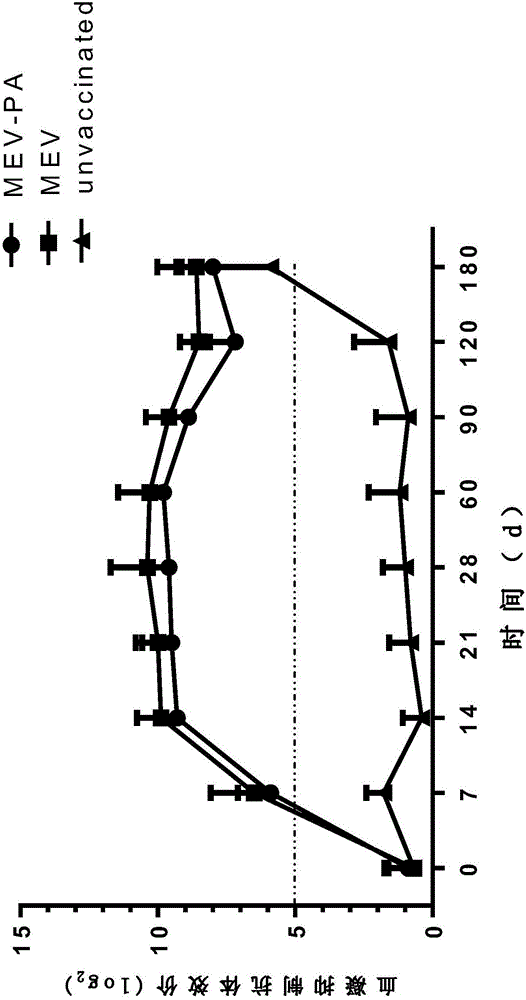
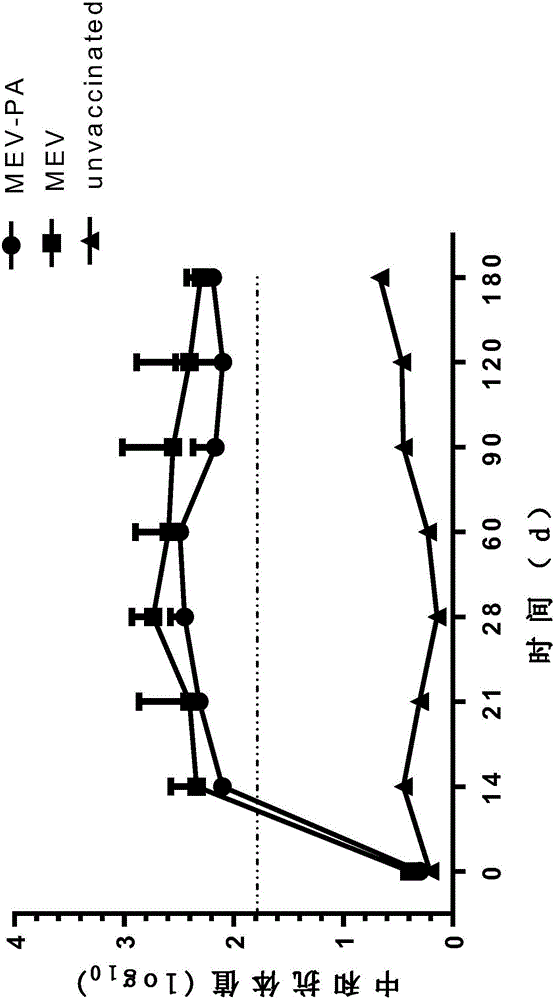
[0156] 1. Efficacy test of MEV-PA dual inactivated vaccine
[0157] 1 immune group
[0158] 1.1 Mink
[0159] Take 120 3-month-old healthy susceptible minks (healthy susceptible mink standard: HI antibody titer in mink parvovirus serum ≤ 1:4, agglutination reaction of Pseudomonas aeruginosa G, B, C, I, F mink serum slides Negative) were randomly divided into 4 groups, 40 in each group, divided into MEV-PA group, MEV group, PA group and control group, respectively intramuscular injection of vaccine 1ml / rat, the control group was injected with the same volume of normal saline in the same way.
[0160] 1.2 Mice
[0161] Fifty mice with a body weight of 16-18 g were divided into 5 groups, 10 mice in each group, and 0.2 ml of the vaccine was injected subcutaneously.
[0162] 2 Evaluation of immune effect on mink hemorrhagic pneumonia
[0163] 2.1 Mink
[0164] 2.1.1 Virus attack protection
[0165] 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com