A kind of avian reticuloendotheliosis virus subunit vaccine injection compound and its preparation method
A subunit vaccine and poultry reticuloendothelial technology, applied in biochemical equipment and methods, viruses, microorganisms, etc., can solve the problems of lack of control effect, few expression products, complicated development process, etc., and achieve good commercialization To develop prospects, prevent or treat infection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 REV immunization antigen
[0041] Specific method: using designed primers
[0042] Upstream primer: 5-CCGGAATTCTCCTCCATACCTACCTATTACA-3', the nucleotide sequence of which is shown in Seq ID No:1;
[0043] Downstream primer: 5-AGAGTCGACTCAATTAGGGAGTGCCGTGT-3, the nucleotide sequence of which is shown in Seq ID No:2;
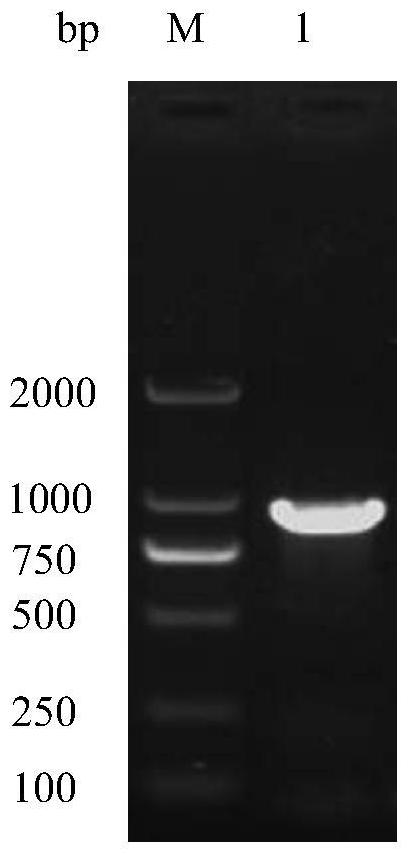
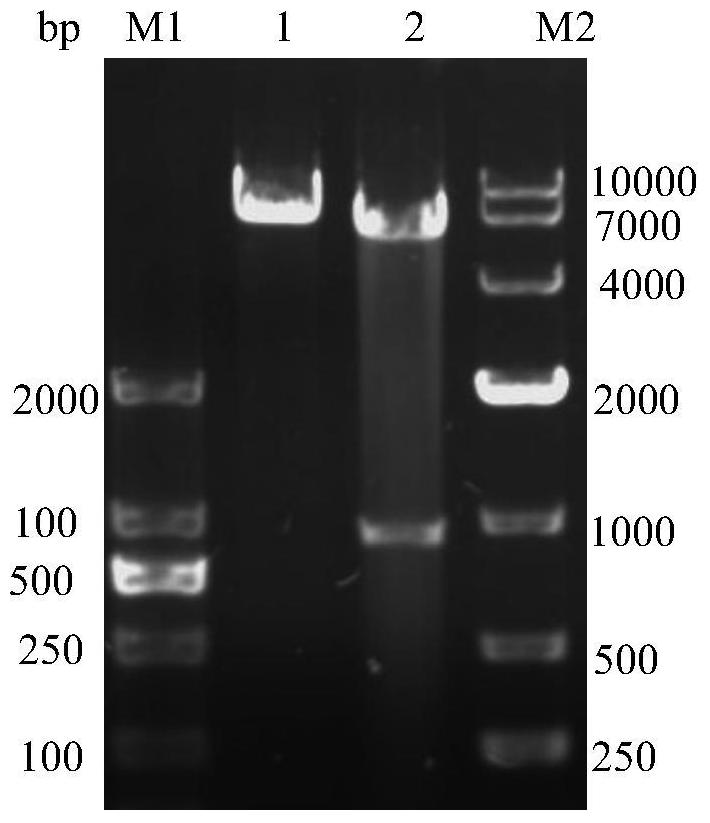
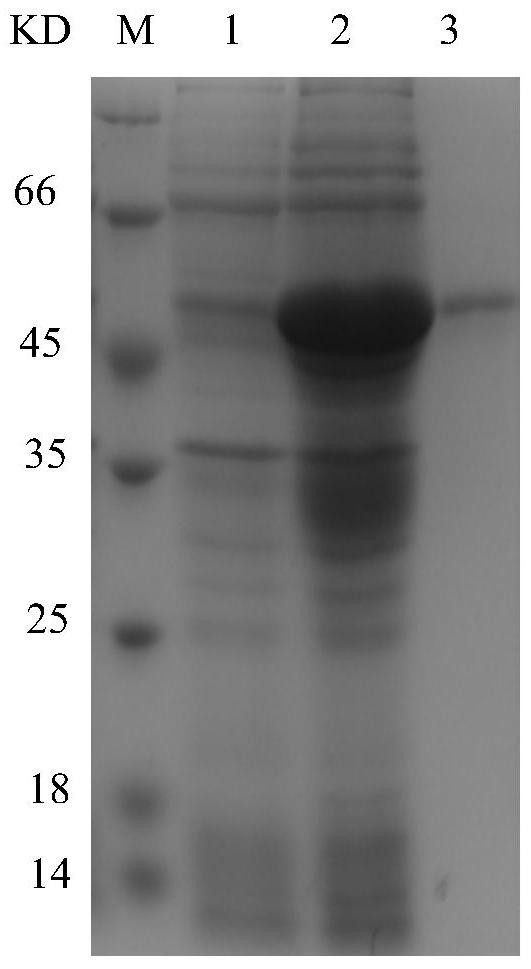
[0044] To amplify the REV-gp90 gene, the steps are: use a DNA extraction kit to extract viral DNA from REV-infected cells, use the DNA as a template, and use the 20 μL PCR reaction system in the Molecular Biology Experiment Manual as a benchmark for the REVgp90 gene Amplification, PCR reaction conditions: 95°C for 3min, make it fully denatured and then enter the cycle system: denature at 95°C for 30s, anneal at 61°C for 30s, extend at 72°C for 70s, cycle 30 times, and finally extend at 72°C for 10min. The amplified product was obtained by nucleic acid electrophoresis test figure 1 The nucleic acid band corresponding to the...
Embodiment 2
[0049] The preparation of embodiment 2 nucleic acid sustained-release adjuvants
[0050] The preparation method of the nucleic acid sustained-release adjuvant is as follows: No. 7 white oil and Siben 80 are mixed at a volume ratio of 94% and 6%, respectively, and then aluminum stearate is added to the above mixture at a mass ratio of 2%, and the mixture is autoclaved at 116°C Bacteria, cooled to 50°C, shake vigorously until transparent;
[0051] Then add all the thioated-modified CpG-ODN at 100 μg / mL, mix well, and prepare an oil emulsion;
[0052] Wherein CpG-ODN is a fully thio-modified 5'-TCGTCGTTTTGTCGTTTTGTCGTT-3' nucleic acid molecule, and its nucleotide sequence is shown in Seq ID No:3.
Embodiment 3
[0053] Example 3 Preparation of Avian Reticuloendotheliosis Virus Subunit Vaccine Injection Complex
[0054] Specific steps are as follows:
[0055] The purified gp90 protein antigen was diluted to 1.0 mg / mL with PBS, then mixed according to the volume ratio of protein antigen:immune adjuvant at 1:1, shaken on the shaker for 1 hour, and emulsified by ultrasonic breaker, ultrasonic emulsified The conditions are: the power is 350W, the working time is 3s, the intermittent time is 2s, the temperature is controlled at 25°C, and the ultrasound is stopped after the emulsification is complete.
[0056] The method for judging the complete emulsification is as follows: Dip the immunogen in the centrifuge tube with a syringe needle, shake the needle to drop the liquid into pre-cooled water, and judge that the emulsification is complete if it does not spread within 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com