Application of type-II cannabinoid receptor agonist in preparation of drugs for treating hypertensive cerebral hemorrhage
A technology of hypertensive cerebral hemorrhage and cannabinoid receptors, which is applied in the field of cerebral hemorrhage drugs to achieve the effects of inhibiting the proliferation and activation of microglia, improving secondary brain damage, and increasing therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
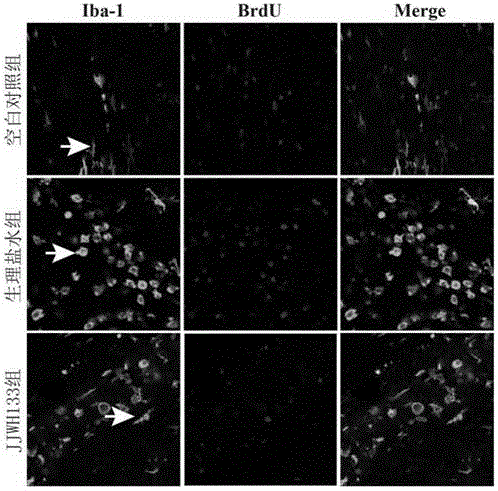
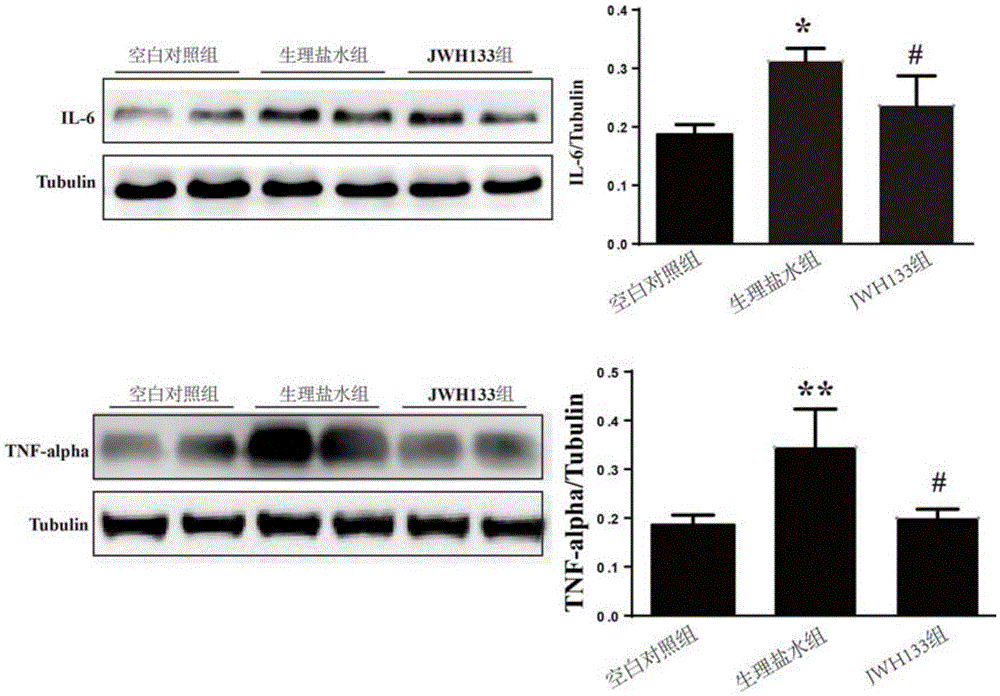
[0042] SD rats (250g-300g) were model animals, and the right femoral artery was dissected and separated, and 200 microliters of autologous arterial blood (without anticoagulant) was taken with a 1ml insulin needle and immediately injected into the right side of the rat through a stereotaxic instrument and a microinjection pump. In the basal ganglia area (slowly inject within 15 minutes), the needle insertion coordinates are 0.2 mm on the mouth side and 2.2 mm on the right side, and the needle tip is inserted 5 mm after breaking through the dura mater. Set up a blank control group (normal SD rats without surgery), a normal saline group (peritoneal injection of the same amount of normal saline after surgery), a JWH133 treatment group (peritoneal injection of JWH133 aqueous solution 1.5 mg / kg, twice a day, 7 days in total), 10 animals in each group.
[0043] Three days after the establishment of the model, the brain was perfused and sliced for immunohistochemistry and brain tis...
Embodiment 2
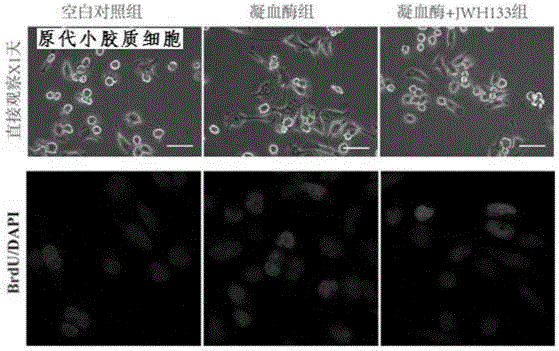
[0049] Effect of Type II Cannabinoid Receptor Selective Agonist (JWH133) on Proliferation of Primary Cultured Microglial Cells Stimulated by Thrombin
[0050] Pregnant 14.5dSD rats were selected, anesthetized intraperitoneally with 5% chloral hydrate, the peritoneum was opened, the embryos were taken out, the scalp, skull and dura mater were removed, and the rat brain was peeled off. Under a dissecting microscope, the olfactory bulb was taken as the center of the sagittal longitudinal section, and the cerebral cortex was collected. Add proliferation medium (DMEM / F12, bFGF20ng / mL, EGF20ng / mL, 2% B27), filter through a 200-mesh filter, count cells after trypan blue staining, and adjust the number of cells to 1×10 6 / ml, transferred to culture flask, put into 5% CO 2 , Cultivated in a constant temperature incubator at 37°C, and passaged once every three days.
[0051] Take the logarithmic growth NSCs, digest with 0.25% EDTA trypsin for 2min, stop the digestion with 10% DMEM / F12...
Embodiment 3
[0054] Minocycline, also known as "minocycline" or "minocycline", is a broad-spectrum antibacterial tetracycline antibiotic with pharmacological properties such as long half-life and lipophilicity.
[0055] Due to the blood-brain barrier in the brain, direct administration of JWH133 is not easy to enter the brain to realize its function. After patients took it, it was found that the effect of JWH133 combined with minocycline was significantly better than that of patients who took JWH133 alone.
[0056] In view of this, the following animal experiments were carried out:
[0057] A rat cerebral hemorrhage model was constructed according to Example 1, and divided into a normal saline control group, a JWH133 alone group and a JWH133+minocycline group, and the number of microglial cells was detected after 7 days of administration. It was found that the number of microglia in the JWH133+minocycline group was 10% less than that in the JWH133 alone group. It can be seen that the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com