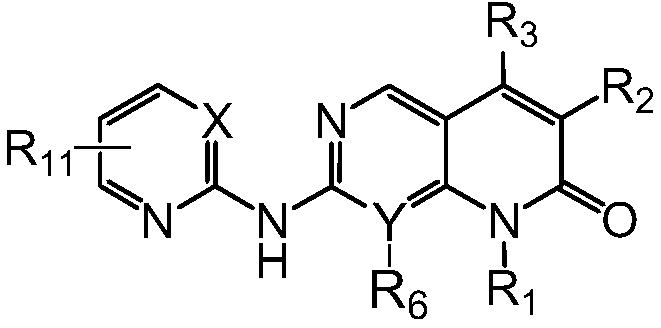
Pyrimidine or pyridopyridone compound and its preparation method and application
A kind of technology of pyridopyridone and compound, applied in the field of pharmaceutical preparation, can solve problems such as lack of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Preparation of Example 1 Compound 1 (prepared according to scheme one line)
[0150] Step 1a: Preparation of 4-cyclopentylamino-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester (4-Cyclopentylamino-2-methylsulfanyl-pyrimidine-5-carboxylic Acid Ethyl Ester) (compound 102): 4 -Chloro-2-methylthio-pyrimidine-5-carboxylic acid ethyl ester (101) (30.06 g, 129 mmol, 1.0 equiv) was dissolved in tetrahydrofuran (300 mL), then cyclopentylamine (12.3 g, mol, 1.1 eq) and triethylamine (39.6 g, 387 mmol, 3.0 eq) were gradually and slowly added to the solution, and the mixture was stirred at room temperature for 14 hours. The precipitated salt was filtered, and the solvent was removed by concentration under reduced pressure. The resulting oil was dissolved in ethyl acetate and washed with saturated aqueous sodium bicarbonate. The organic phase was dried over sodium sulfate. The salt was filtered, and the solvent was removed by concentration under reduced pressure to obtai...
Embodiment 2
[0166] Preparation of Example 2 Compound 3 (prepared according to scheme one line)
[0167] The preparation of compound 110-3 is as in step 1i in Example 1, except that 2-benzyloctahydropyrrolyl[3,4-c]pyrrole is replaced by 2.5-bicyclo[2.2.1]heptane- 2 – tert-butyl formate, prepared to obtain tert-butyl 5-(6-aminopyridin-3-yl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate (tert-butyl5- (6-aminopyridin-3-yl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate) (compound 110-3). Target product characterization data: LCMS(ESI):m / z 291[M+1] + .
[0168] The preparation of compound 111-3, the synthesis method is the same as 1j in Example 1, only the compound 110-1 is replaced by 110-3, and 5-(6-((6-bromo-8-cyclopentyl- 5-methyl-7-oxo-7,8-dihydropyrimidin[2,3-d]-2-pyrimidine)amine)-3-pyridine)-2,5-diazabicyclo[2.2.1] Heptane-2-carboxylic acid tert-butyl ester (tert-butyl5-(6-((6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidin-2 -yl)amino)pyridin-3-yl)-2,5-diazabicy...
Embodiment 3
[0171] Preparation of Example 3 Compound 2 (prepared according to scheme two lines)
[0172] Step 3a: Preparation of 5-iodine-2-chloro-4-cyclopentylaminopyrimidine (5-iodine-2-chloro-4-cyclopentyl-aminopyrimidine) (compound 202): 2,4-dichloro-5- Iodopyrimidine (Compound 201) (80.00 g, 0.291 mol, 1.0 eq) was dissolved in ethanol (800 ml), triethylamine (88.18 g, 0.873 mol, 3.0 eq) was added, and then the mixture was stirred under an ice bath, When the temperature dropped to 0°C-5°C, cyclopentylamine (49.50 g, 0.582 mol, 2.0 equivalents) was added dropwise, and the dropwise addition was completed in 30 minutes, and then kept stirring at about 5°C. HPLC monitoring, when the peak area ratio of 2,4-dichloro-5-iodopyrimidine is less than 1%, the reaction is terminated. The reaction solution was concentrated, diluted with ethyl acetate (300 mL), added with water (300 mL), extracted and separated. The aqueous phase was extracted with ethyl acetate (200 mL x 2). The organic phases w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com