Novel medical application of isopentenyl isoflavone compounds in licorice roots
A technology of compounds and mixtures, which is applied in the field of new medical applications of a class of prenyl isoflavones in licorice, and can solve the problems that the related activities of the three compounds have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
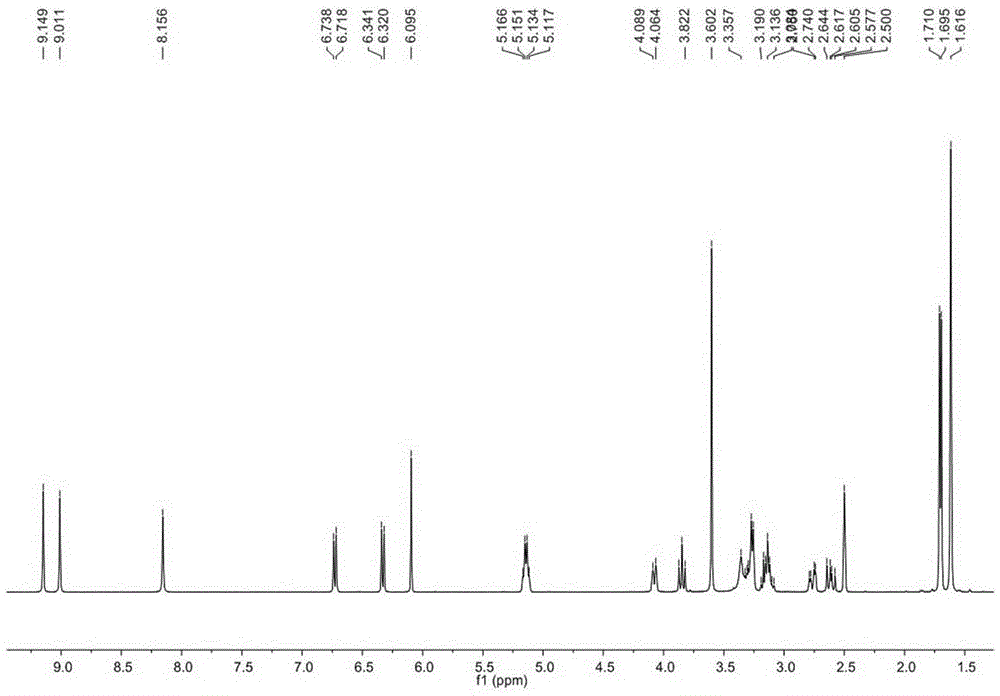
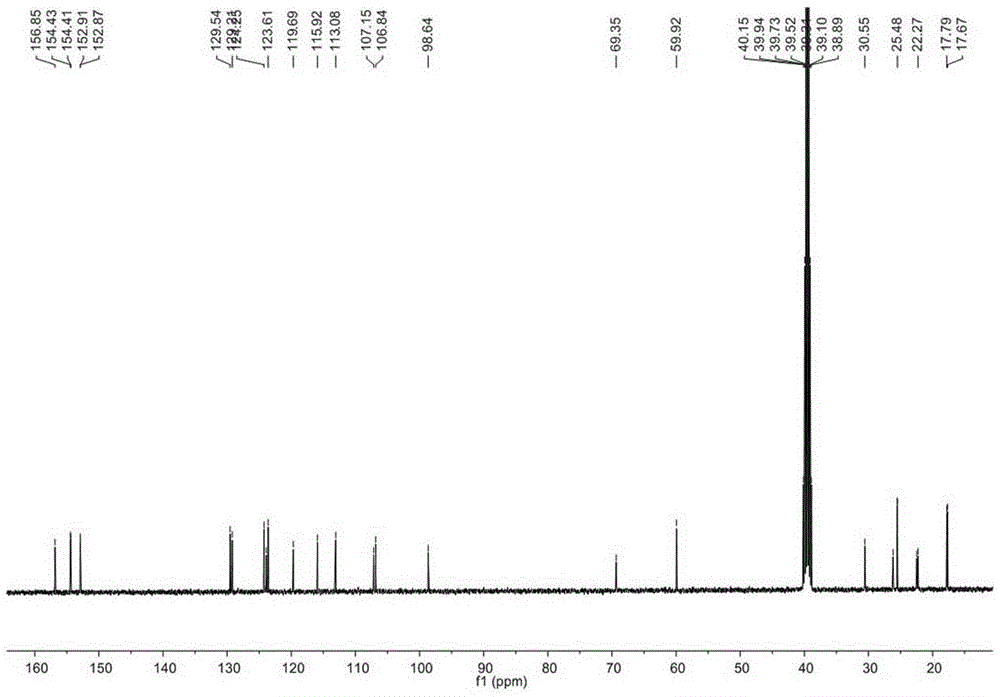
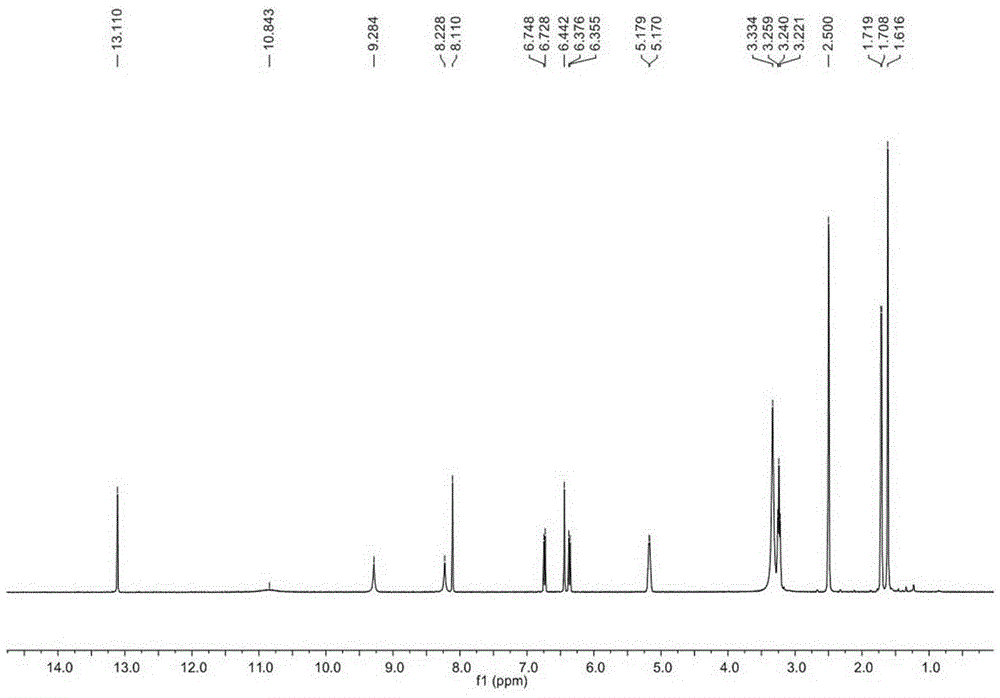
[0028] Example 1. Separation and purification of Glycyrrhizidine, angustoneA and crude Liquiritigenin D.
[0029] 1. Experimental materials and methods.
[0030] All chemical reagents mentioned in this method were purchased from Beijing Chemical Plant.
[0031] Get 35kg of licorice medicinal material (Glycyrrhiza branch of Inner Mongolia Yili Science and Technology Industrial Co., Ltd.), pulverize, extract twice with 10 times the amount of 95% ethanol and 70% ethanol, each time for 2 to 3 hours, reclaim the ethanol under reduced pressure, and the obtained The extract (10 L) was suspended in water and extracted 5 times with ethyl acetate to obtain 2500 g of the ethyl acetate fraction. Take 1280g of ethyl acetate, separate it by silica gel column chromatography (200-300 mesh, Qingdao Ocean Chemical Co., Ltd.), and elute with petroleum ether-ethyl acetate gradient (1:0-1:1) to obtain fractions A-J . Fraction E was separated by MCI column chromatography (Chengdu Kepu Biological C...
Embodiment 2
[0034] Example 2, Glycyrrhizidine, angustoneA and pillycyrrhizin D inhibit the viability of various cancer cells.
[0035] 1. Experimental materials and methods.
[0036] HepG2 human liver cancer cells, SW480 human colon cancer cells, A549 human lung cancer cells, and MCF7 human breast cancer cells were purchased from the American Type Culture Collection (ATCC), and all experiments used cells in logarithmic growth phase.
[0037] (1) 12 hours after the cells were seeded in a 96-well plate, add the specified licorice compound to a final concentration of 10 μM, and continue to cultivate for 24 hours, then add MTS solution to each well to a final concentration of 0.5 mg / mL, and continue to cultivate for 2 to 4 hours. Hours, and then use an automatic microplate reader to measure the absorbance of each well at 490nm, and calculate its inhibition rate relative to the control group.
[0038] (2) 12 hours after the cells were seeded in the 96-well plate, add the specified licorice co...
Embodiment 3
[0043] Example 3, Glycyrrhizin and angustoneA inhibit SW480 cell proliferation and induce SW480 cell apoptosis.
[0044] 1. Experimental materials and methods.
[0045] (1) After SW480 cells were seeded in 6-well plates for 12 hours, add Glycyrrhizidine or angustoneA to the specified concentration, continue to culture for 24 hours, collect all floating and adherent cells, add 1 mL of 70% ethanol, and fix overnight at -20°C . Centrifuge the fixed cells at 200 g for 5 minutes, discard the supernatant, add 1 mL of PBS (Zhongke Maichen Technology Co., Ltd.), pipette evenly, and centrifuge again. Then add 5 μg / mL propidium iodide (PI) and 100 μg / mL RNase (Beijing Boya Innovation Technology Development Co., Ltd.), incubate at 37°C for 30 minutes, and use FASCAN flow cytometry (Beckon Dickenson Company, USA) to measure, The excitation wavelength is 488nm and the emission wavelength is 630nm.
[0046] (2) 12 hours after SW480 cells were inoculated in a 6-well plate, licoricedin or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com