PTP1B inhibitors and synthesis thereof, and application of PTP1B inhibitors to preparation of medicines for treating type 2 diabetes
An inhibitor and compound technology, which can be used in the preparation of carbon-based compounds, the preparation of organic compounds, and the preparation of ethers by dehydration of hydroxyl-containing compounds, which can solve the problems of high electronegativity, poor selection specificity, and not much.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
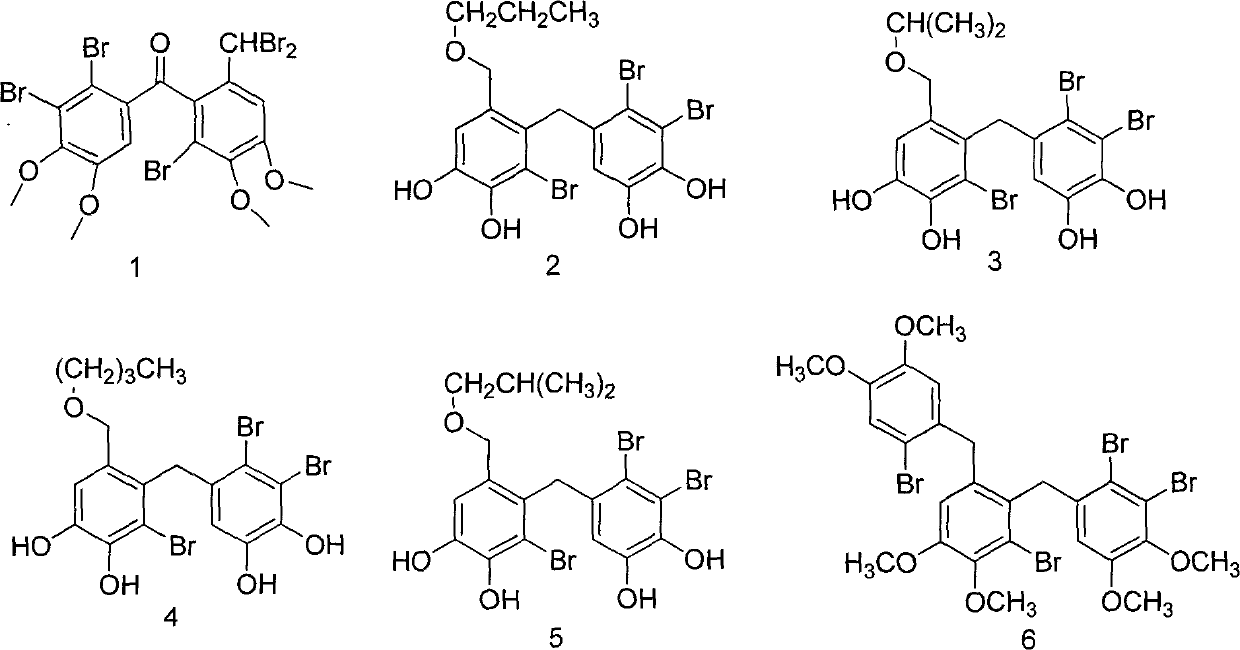
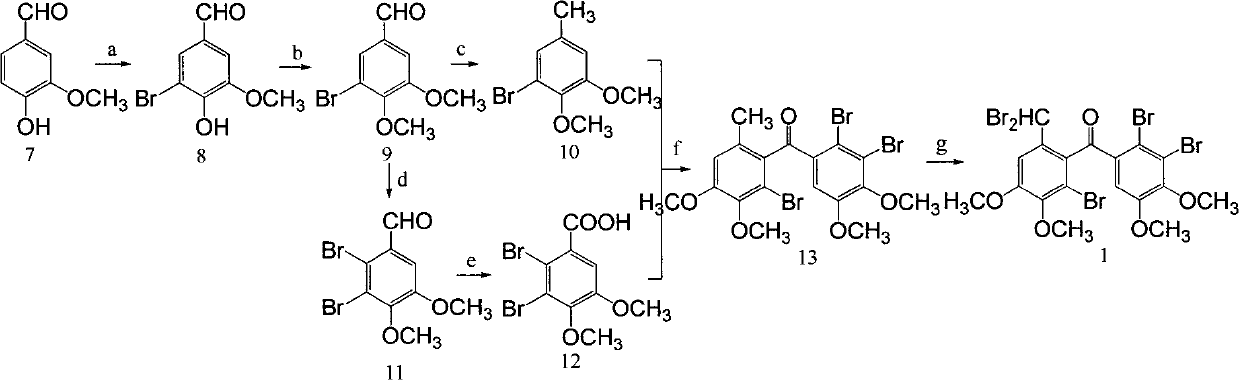
[0074] Example 1 "(2'-bromo-6'-dibromomethyl-3',4'-dimethoxyphenyl)-(2,3-dibromo-4,5-dimethoxyphenyl )-Methanone"Chemical Total Synthesis and Structure Identification
[0075] (1) Chemical Synthesis and Structure Identification of 5-Bromovanillin
[0076] At 0°C, add 2.8ml of Br dropwise to 60ml of methanol dissolved with 7.6g (0.05mol) of vanillin 2 , 2 hours to complete the addition, stirred at room temperature for 1 hour, then added dropwise 25ml of water (precipitation) at 0°C, added 20 minutes, continued to stir for 15 minutes, filtered the precipitate, washed the precipitate with ice water, and drained to obtain 10.7g of white crystals, after spectral analysis, it was confirmed that the compound was 5-bromovanillin;
[0077] The physical and chemical properties of the compound are as follows: white crystal, melting point 160-162°C; H NMR spectrum: 1 HNMR (500Hz, CDCl 3 ): δ9.78(s, 1H), 7.64(d, J=1.65, 1H), 7.36(d, J=1.65, 2H), 6.53(s, 1H), 3.98(s, 3H); NMR carbon Sp...
Embodiment 2
[0096] Example 2 "3,4-dibromo-5-[(2'-bromo-3',4'-dihydroxy-6'-n-propoxymethyl-phenyl)-methyl]-1,2 - Hydroquinone, 3,4-dibromo-5-[(2'-bromo-3',4'-dihydroxy-6'-isopropoxymethyl-phenyl)-methyl]-1, 2-benzenediol, 3,4-dibromo-5-[(2'-bromo-6'-n-butoxymethyl-3',4'-dihydroxy-phenyl)-methyl]-1 , 2-benzenediol, 3,4-dibromo-5-[(2′-bromo-3′,4′-dihydroxy-6′-isobutoxymethyl-phenyl)-methyl]- Chemical synthesis and structure identification of 1,2-benzenediol"
[0097] (1) Chemical synthesis and structure identification of 5,6-dibromo-3,4-dimethoxybenzyl alcohol
[0098] Under the ice-water bath, 2.2 grams of sodium borohydride were added to 72 grams of 5,6-dibromoveratraldehyde in the methanol (400ml) solution, stirred, and detected by TLC. After the raw material point disappeared, 10% of Dilute hydrochloric acid until the solution is weakly acidic, distill off methanol, extract the obtained solid with equal volumes of dichloromethane and water (200ml each), dry the organic phase with anhy...
Embodiment 3
[0118] Example 3 "2,3-dibromo-1-(2'-bromo-6'-(2"-bromo-4",5"-dimethoxybenzyl)-3',4'-di Chemical synthesis and structure identification of methoxybenzyl)-4,5-dimethoxybenzene"
[0119] (1) 2,3-dibromo-1-(2'-bromo-6'-(3",4"-dimethoxybenzyl)-3',4'-dimethoxybenzyl )-4,5-Dimethoxybenzene Chemical Synthesis and Structure Identification
[0120] The compound veratrole 0.69g and 3-bromo-2-[(2′,3′-dibromo-4′,5′-dimethoxy)-benzyl]-4,5-methoxybenzene Dissolve 2.78g of methanol in dichloromethane, add 0.8g of aluminum trichloride to the above mixed solution under ice-water bath conditions, detect by TLC, after the raw material point disappears, pour the reaction solution into ice water, extract and separate, organic The phase was washed 3 times with dilute hydrochloric acid with a mass concentration of 3%, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and recrystallized from methanol to obtain 2.8 g of a white solid, which was confirmed to be 2,3-dibromo-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com