Novel sulfonamide compound, preparation method, and use of novel sulfonamide compound as protein tyrosine phosphatase 1B inhibitor
A compound and inhibitor technology, applied in the preparation of sulfonamides, organic chemistry, drug combination, etc., can solve problems such as poor selectivity, poor cell permeability and bioavailability, and easy ionization of proton-containing acid fragments in compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
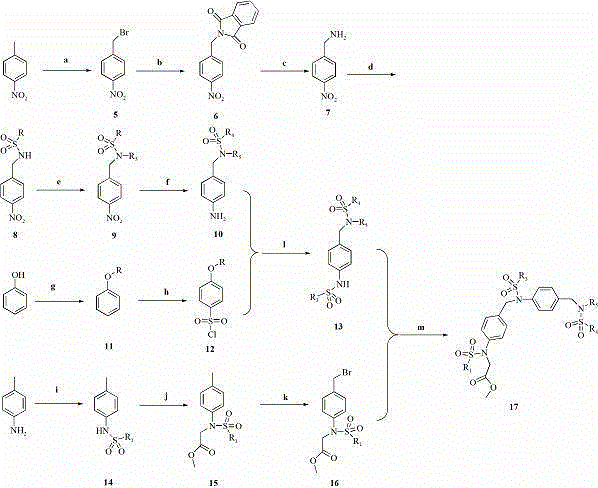
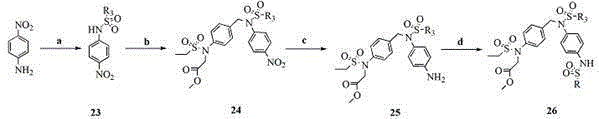
[0037]Example 1: [[4-[[Benzenesulfonyl[4-[(benzylmethanesulfonamido)methyl]phenyl]amino]methyl]phenyl]methanesulfonamido]acetate methyl ester (P1) Synthetic specific preparation method: Reaction step a: Place the starting material p-nitrotoluene (15 g, 110 mmol) in a 250 mL three-necked round-bottomed flask, add carbon tetrachloride (100 mL) to dissolve and heat with stirring, until the temperature When it was raised to about 75°C, NBS (19.58 g, 110 mmol) was added in batches, and the stirring was continued for 7 hours. TLC followed the progress of the experiment until the reaction was complete. After the reaction was completed, filter, wash the solid with carbon tetrachloride (100 mL), and distill the filtrate under reduced pressure, dissolve it with ethyl acetate, wash with saturated brine (2×100 mL), dry over anhydrous Na2SO4, and remove the solvent by distillation under reduced pressure . The obtained solid was recrystallized with ethanol, and a pale yellow needle-like so...
Embodiment 2
[0038] Example 2. [[4-[[[4-[(Benzylmethanesulfonamido)methyl]phenyl]-(4-methoxybenzenesulfonamido)]methyl]phenyl]methanesulfonamide Synthesis of methyl] acetate (P2) R 6 Reaction step g: Dissolve the starting material in p-phenol (3 g, 32 mmol) and place in a 100 mL round bottom flask, add methanol to dissolve, then add KOH (2.69 g, 48 mmol) and stir, nitrogen protection Dimethyl sulfate (6.06 g, 48 mmol) was added at 0°C to continue stirring, and then warmed to room temperature for reaction. After reacting for 5 hours, TLC detected that the reaction was complete, cooled to room temperature, distilled off methanol under reduced pressure, added ethyl acetate (80 mL) to the reaction solution, and washed the organic phase with saturated brine (3×100 mL), and washed the organic phase with Anhydrous NaSO 4 dry. The organic solvent was distilled off under reduced pressure, and the resulting product anisole was 1.94 g. Yield: 56%. Reaction step h: the above-mentioned compound ani...
Embodiment 3
[0039] Example 3. [[4-[[[4-[(Benzylmethanesulfonylamino)methyl]phenyl]-(4-ethoxybenzenesulfonylamino)]methyl]phenyl]methanesulfonamide Synthesis of methyl] acetate (P3) R 6 Be ethyl; Reaction step 2a (preparation of compound 2B): dissolving starting material in p-phenol (1.88 g, 20 mmol) is placed in 100mL round bottom flask, adds DMF to dissolve, then adds K 2 CO 3 (4.14 g, 30 mmol) was heated and stirred at 70°C, and bromoethane (2.3 mL, 30 mmol) was added after 30 min to continue stirring, and then warmed to room temperature for reaction. After reacting for 3 hours, after the reaction was complete as detected by TLC, cool to room temperature, filter the reaction solution and wash with ethyl acetate (2 × 70 mL), combine the organic phases and wash with saturated brine, separate the organic phases and wash with anhydrous Na 2 SO 4 Drying, distillation under reduced pressure to remove the solvent and column chromatography yielded 1.72 g of pure phenetole: 70%; then refer to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com