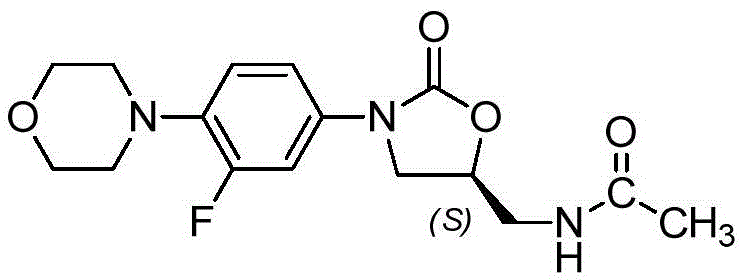
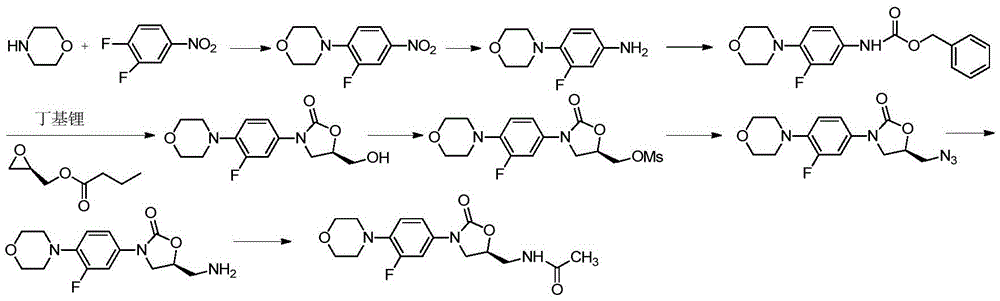
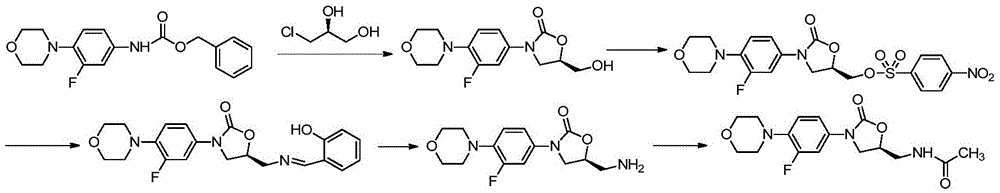
Preparing method for linezolid and intermediate thereof
A technology of linezolid and its compounds, applied in the fields of organic chemistry, organic chemistry, etc., can solve the problems of insufficient optical purity of linezolid and its intermediates, low total yield of synthetic routes, unsuitability for industrial production, etc., to avoid Inflammable, explosive and toxic reagents, easy operation, mild reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the preparation of compound B
[0047]
[0048] In a 500ml autoclave under a hydrogen atmosphere, add compound A: ethyl 4-chloroacetoacetate (50g, 0.30mol), 5ml hydrochloric acid (AR, content 36% to 38%), 300ml methanol, stir the reaction solution to dissolve, add Chiral ruthenium metal catalyst (R)-RuCl 2 (BINAP) (50mg), the reaction liquid was replaced with hydrogen twice, the pressure was raised to 8-10 atmospheres, and the temperature was kept at 95-98°C for 1-2 hours. The reaction was cooled to room temperature and analyzed directly by GC to measure the conversion (column: HP-10125m / 0.2mm) and enantiomeric excess (column: Lipodex-E25m / 0.25mm). The enantiomeric excess was 99.95% and the conversion was 100%. After the filtrate was dried and filtered, the solvent was distilled off under reduced pressure to obtain compound B, which was directly used in one-step reaction.
Embodiment 2
[0049] Embodiment two, the synthesis of compound C
[0050]
[0051] The compound B obtained in Example 1 was dissolved in 200ml of dichloromethane, sodium carbonate (37.2g, 0.35mol) was added to the reaction solution to cool down to 0-5°C, acetyl chloride (23.5g, 0.30mol) was added dropwise, and the reaction was kept for 5h , 200ml of water was added, the organic phase was washed successively with water and saturated brine, dried and filtered, and the solvent was distilled off under reduced pressure to obtain 53.5 g (0.256 mol) of the crude product of Compound C, with a molar yield of 95%.
Embodiment 3
[0052] Embodiment three, the synthesis of compound D
[0053]
[0054] Compound C (53.5 g, 0.256 mol) synthesized in Example 2 was dissolved in 200 ml of dichloromethane, 3-fluoro-4-morpholinoaniline (compound IV, 52 g, 0.265 mol) was added, stirred at room temperature to dissolve, and added Sodium carbonate (35.3g, 0.33mol), keep stirring at room temperature for 4 to 8 hours, TLC detects that the reaction is complete, add 200ml of water and stir for 1h, the organic phase is washed with water in turn, washed with saturated saline, dried and filtered, and the solvent is distilled off under reduced pressure to obtain intermediate Body D84g (0.23mol), molar yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com