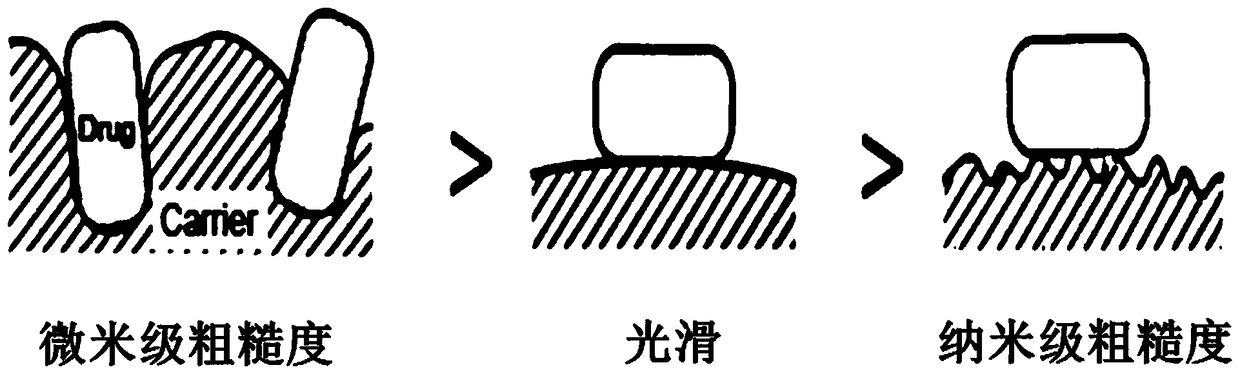
Particles with nano-scale rough structure on the surface and its preparation method and application
A rough structure, nano-scale technology, applied in non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as poor product stability, demanding condition control, and poor particle fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Dissolve lactose, mannitol, trehalose, and chitosan in water respectively, and prepare solutions with a concentration of 20 mg / ml respectively, and then use a spray dryer to spray dry the above solutions to prepare single carrier particles. The parameters of spray drying are as follows: The air temperature is 140°C, the outlet air temperature is 87°C, the pump liquid rate is 7ml / min, the nozzle diameter is 0.71mm, the atomization pressure is 150Kpa, and the air flow rate is 0.60m 3 / h.
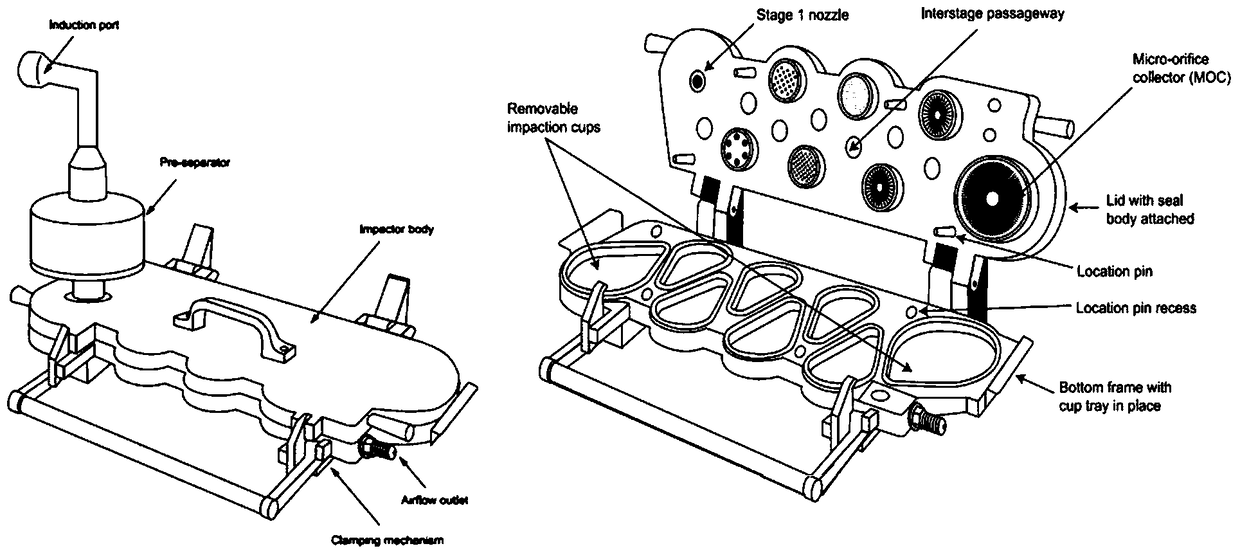
[0046] The spray-dried carrier granules are mixed with the micronized venlafaxine drug granules at a mass ratio of 20:1, and packed into No. 3 capsules (10 ± 0.5 mg / capsule), to obtain the venlafaxine dry powder inhaler. Next Generation Pharmaceutical Impactor (NGI, figure 2 ) to evaluate the in vitro drug deposition rate of venlafaxine dry powder inhalation.
[0047] Detection method: Take 1 capsule of the test product of the above-mentioned venlafaxine dry powder inhaler, and put it ...
Embodiment 2
[0053] Dissolve any two of lactose, mannitol, trehalose and chitosan in water at a mass ratio of 50:50 to prepare solutions with a concentration of 20 mg / ml, and then use a spray dryer to spray dry the above solutions to prepare Binary carrier particles, the parameters of spray drying are as follows: the air inlet temperature is 140°C, the air outlet temperature is 87°C, the pump liquid rate is 7ml / min, the nozzle diameter is 0.71mm, the atomization pressure is 150Kpa, and the air flow rate is 0.60m 3 / h.
[0054] The carrier particle after the spray drying is prepared venlafaxine dry powder inhaler by the method for embodiment 1, adopts new-generation pharmaceutical collider to measure the drug effective deposition rate of venlafaxine dry powder inhaler (detection method is the same as embodiment 1) , the results are shown in Table 1: the binary carrier particles prepared from mannitol and chitosan had the highest effective drug deposition rate, reaching 49.14%.
Embodiment 3
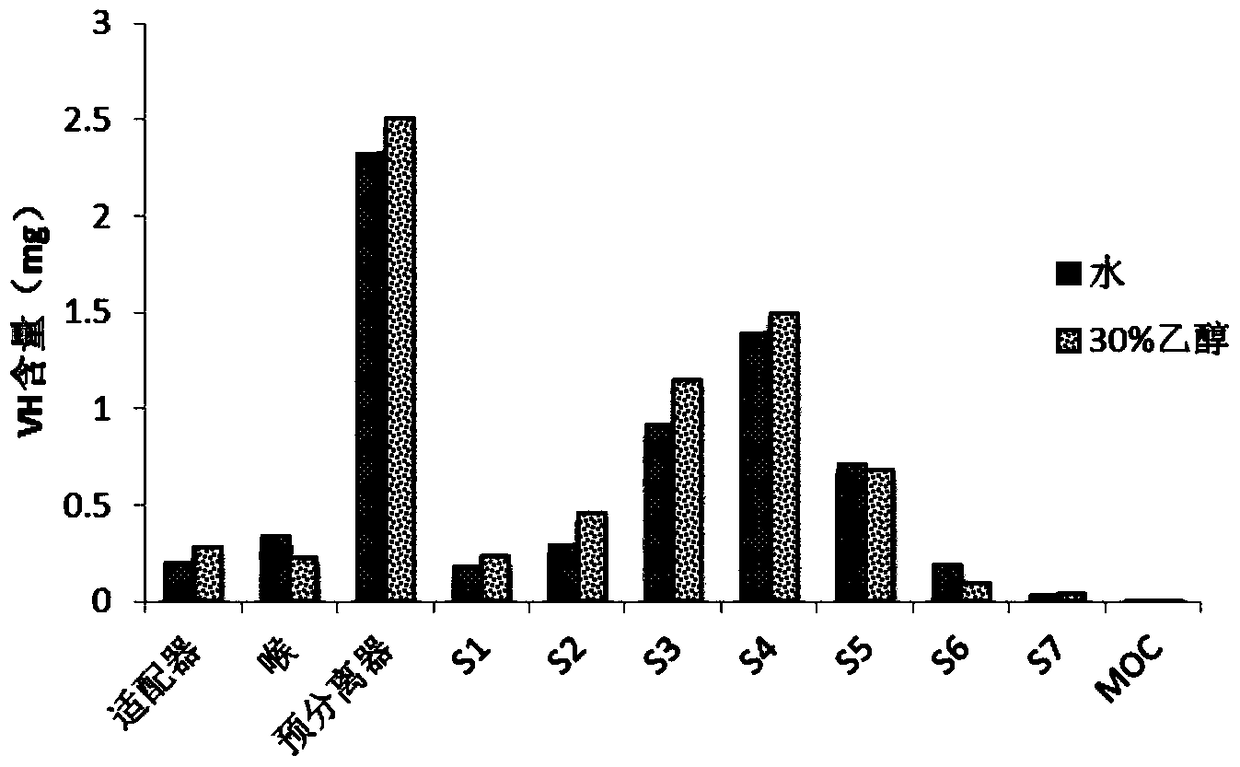
[0056] Dissolve mannitol and chitosan with a mass ratio of 50:50 in ethanol or water with a volume fraction of 30%, and prepare solutions with a concentration of 20 mg / ml respectively, and then use a spray dryer to spray dry the above solutions to prepare binary Carrier particles, spray drying parameters are as follows: air inlet temperature is 140°C, air outlet temperature is 87°C, pump liquid rate is 7ml / min, nozzle diameter is 0.71mm, atomization pressure is 150Kpa, air flow rate is 0.60m 3 / h.
[0057] The carrier particle after the spray drying is prepared venlafaxine dry powder inhaler by the method for embodiment 1, adopts the drug effective deposition rate of venlafaxine dry powder inhaler to measure venlafaxine dry powder inhaler by adopting new generation (detection method is the same as embodiment 1 ). The result is as image 3 It is shown that the carrier particle prepared by using 30% ethanol as the solvent has a higher effective drug deposition rate than the pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com