Method for separating and analyzing alogliptin benzoate and its related substance
A technology for separation and analysis of related substances, applied in the field of chemical analysis, can solve the problems of poor reproducibility of impurities, inaccurate integration, influence on the correct evaluation of preparation substances, etc., and achieve the effect of good elution separation effect and reliable separation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
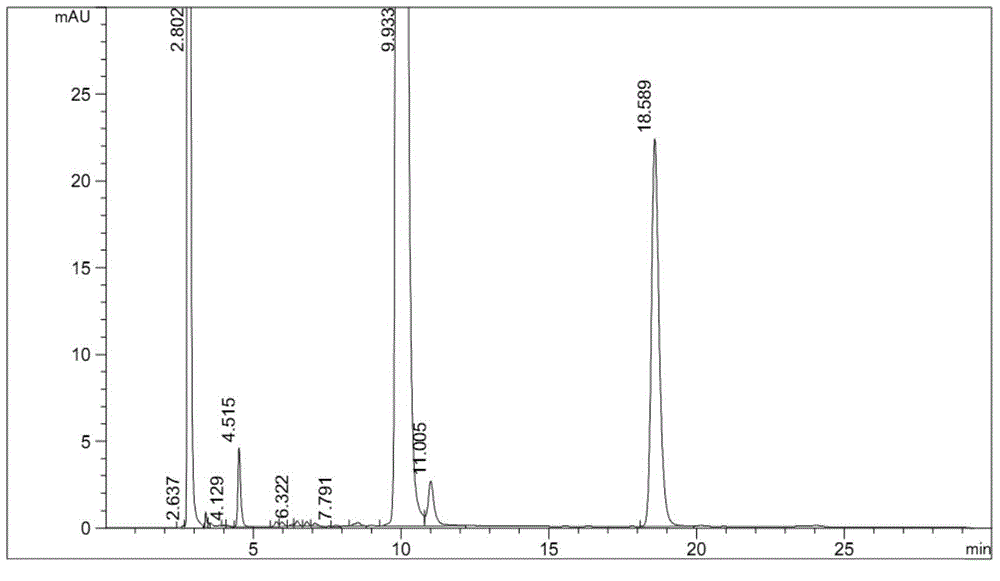
Embodiment 1
[0043] Instrument: Agilent1260 high performance liquid chromatography, DAD detector
[0044] Chromatographic column: Octyl bonded silica gel as filler (4.6mm×250mm, 5μm)
[0045] Mobile phase A: 0.04mol / L potassium dihydrogen phosphate buffer (containing 0.3% by volume of triethylamine), adjust pH to 3.0.0 with phosphoric acid
[0046] Mobile Phase B: Acetonitrile
[0047] See the table below for gradient elution:
[0048]
[0049] Flow rate: 1.0mL / min
[0050] Wavelength: 278nm
[0051] Column temperature: 30°C
[0052] Injection volume: 10μL
[0053] Diluent: mobile phase A
[0054] Experimental procedure: Take 20 tablets of the test product, weigh them accurately, calculate the average tablet weight, and grind them finely. Precisely weigh 30 mg fine powder equivalent to alogliptin into a 100 mL volumetric bottle, add 6 mL of 30% hydrogen peroxide solution to wet the sample for oxidative degradation, leave it at room temperature for 6 hours, add diluent to ultrason...
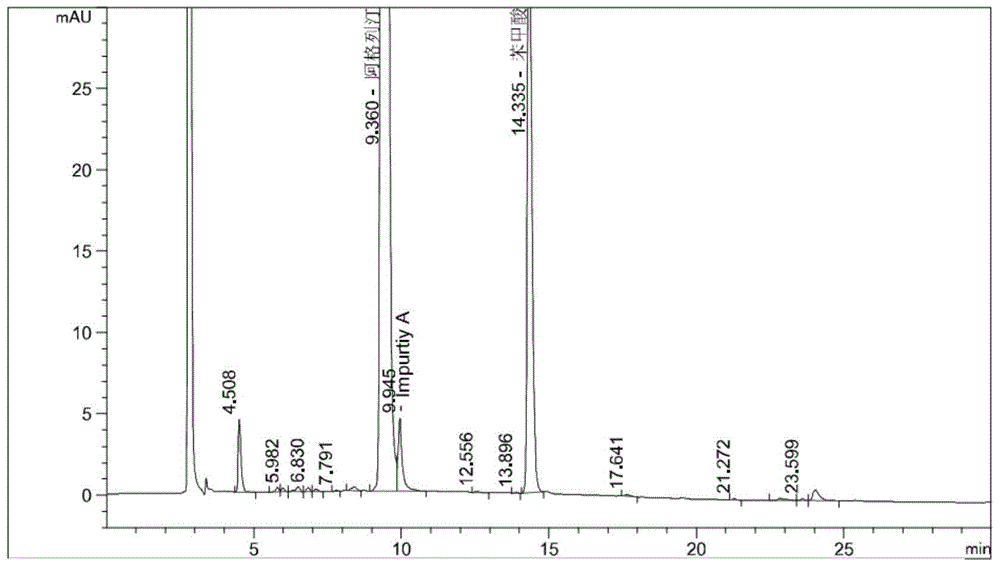
Embodiment 2
[0058] Instrument: Agilent1260 high performance liquid chromatography, DAD detector
[0059] Chromatographic column: Octyl bonded silica gel as filler (4.6mm×250mm, 5μm)
[0060] Mobile phase A: 0.04mol / L sodium dihydrogen phosphate buffer solution (containing 0.3% by volume of triethylamine), adjust the pH to 3.0 with phosphoric acid
[0061] Mobile Phase B: Acetonitrile
[0062] See the table below for gradient elution:
[0063]
[0064] Flow rate: 1.0mL / min
[0065] Wavelength: 278nm
[0066] Column temperature: 30°C
[0067] Injection volume: 10μL
[0068] Diluent: mobile phase A
[0069] Experimental procedure: Take 20 tablets of the test product, weigh them accurately, calculate the average tablet weight, and grind them finely. Precisely weigh approximately 30 mg of alogliptin fine powder into a 100 mL volumetric bottle, add 6 mL of 30% hydrogen peroxide solution to wet the sample for oxidative degradation, leave it at room temperature for 6 hours, add buffer s...
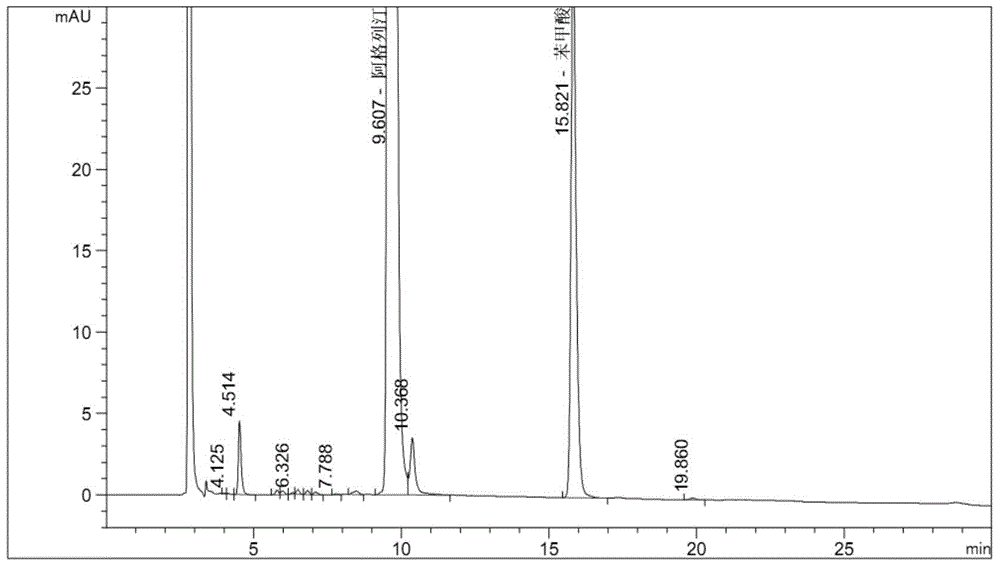
Embodiment 3
[0072] Instrument: Agilent1260 high performance liquid chromatography, DAD detector
[0073] Chromatographic column: Octyl bonded silica gel as filler (4.6mm×250mm, 5μm)
[0074] Mobile phase A: 0.04mol / L potassium dihydrogen phosphate buffer solution (containing 0.3% by volume of triethylamine), adjust the pH to 3.0 with phosphoric acid
[0075] Mobile Phase B: Acetonitrile
[0076] See the table below for gradient elution:
[0077]
[0078] Flow rate: 1.0mL / min
[0079] Wavelength: 278nm
[0080] Column temperature: 30°C
[0081] Injection volume: 10μL
[0082] Diluent: mobile phase A
[0083] Experimental procedure: Take 20 tablets of the test product, weigh them accurately, calculate the average tablet weight, and grind them finely. Precisely weigh approximately 30 mg of alogliptin fine powder into a 100 mL volumetric bottle, add 6 mL of 30% hydrogen peroxide solution to wet the sample for oxidative degradation, leave it at room temperature for 6 hours, add buffe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com