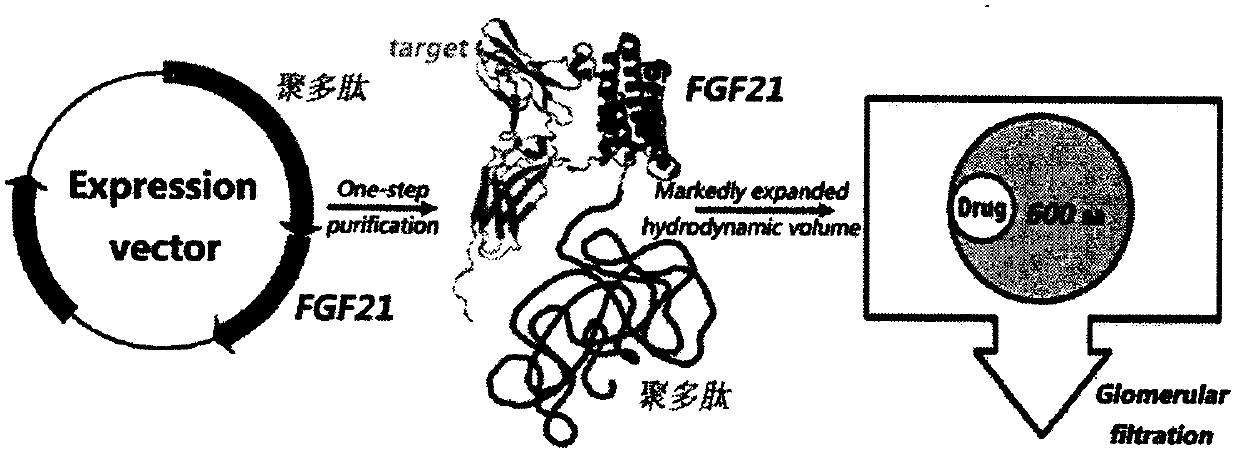
Recombinant polypeptide and its application
A polypeptide and amino acid technology, applied in the direction of peptides, desipeptides, hybrid peptides, etc., can solve the problems of reducing affinity with target receptors, complex and large screening process, affecting drug tissue distribution, etc., to increase the hydrodynamic volume , reduce immunogenicity, and reduce the effect of screening workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
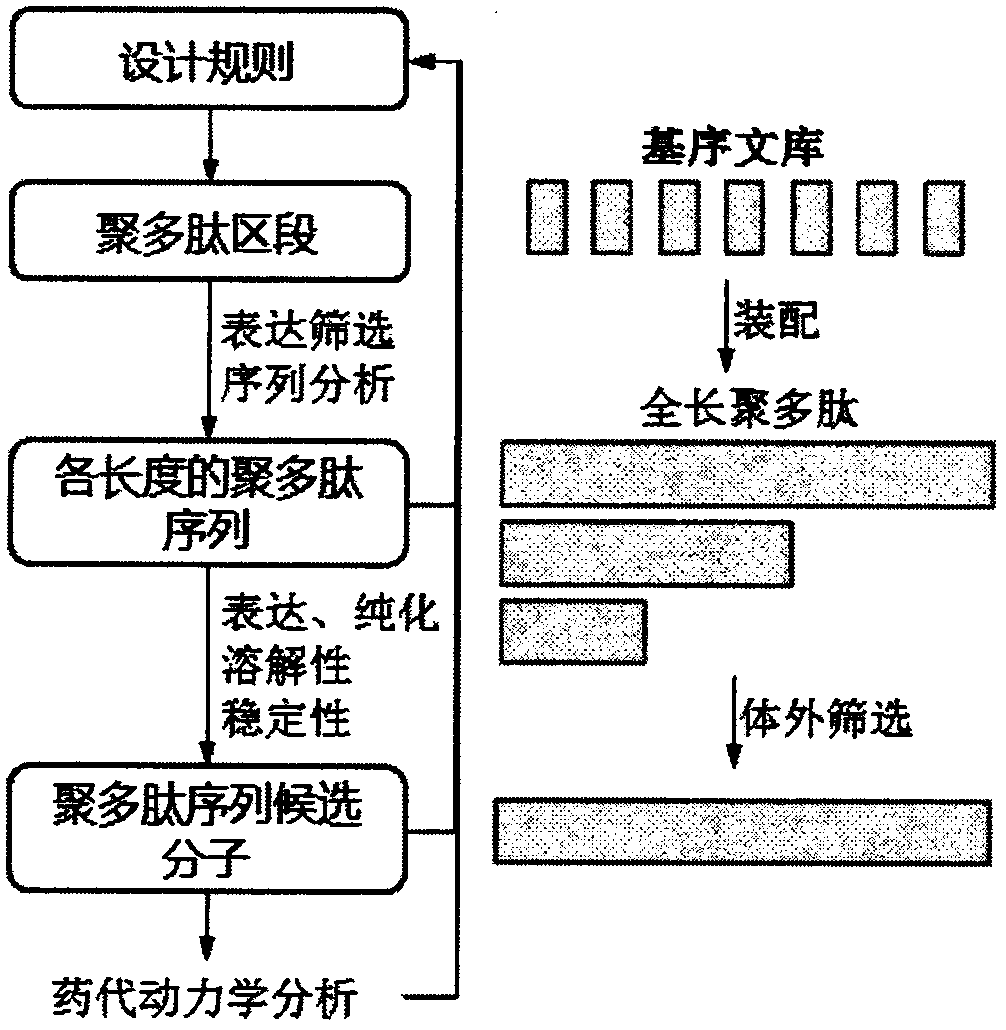
[0057] Example 1: Design and Construction of Short Sequences
[0058] The following examples take the design and construction of a short sequence with a length of 10 amino acids as an example. 3-6 kinds of glycine (G), alanine (A), serine (S), threonine (T), proline (P) and lysine (K) constituting the short sequence were respectively selected type amino acids, and none of the amino acid residues will appear consecutively in the short sequence. For example, three types of amino acids, alanine, serine, and lysine, were selected, and 10 peptides containing 10 amino acids were designed, named sequence library PT01 (named after the acronym PT of Polypeptide Tag, polypeptide library, Polypeptides are named after P), the sequence names of the amino acid and nucleotide sequences and the SEQ ID NOs of these segments are listed in Table 1. For example, four types of amino acids, glycine, serine, proline, and lysine, were selected, and 10 peptides containing 10 amino acids were designe...
Embodiment 2
[0067] Example 2: Construction of a short sequence segment of 20 amino acids in length
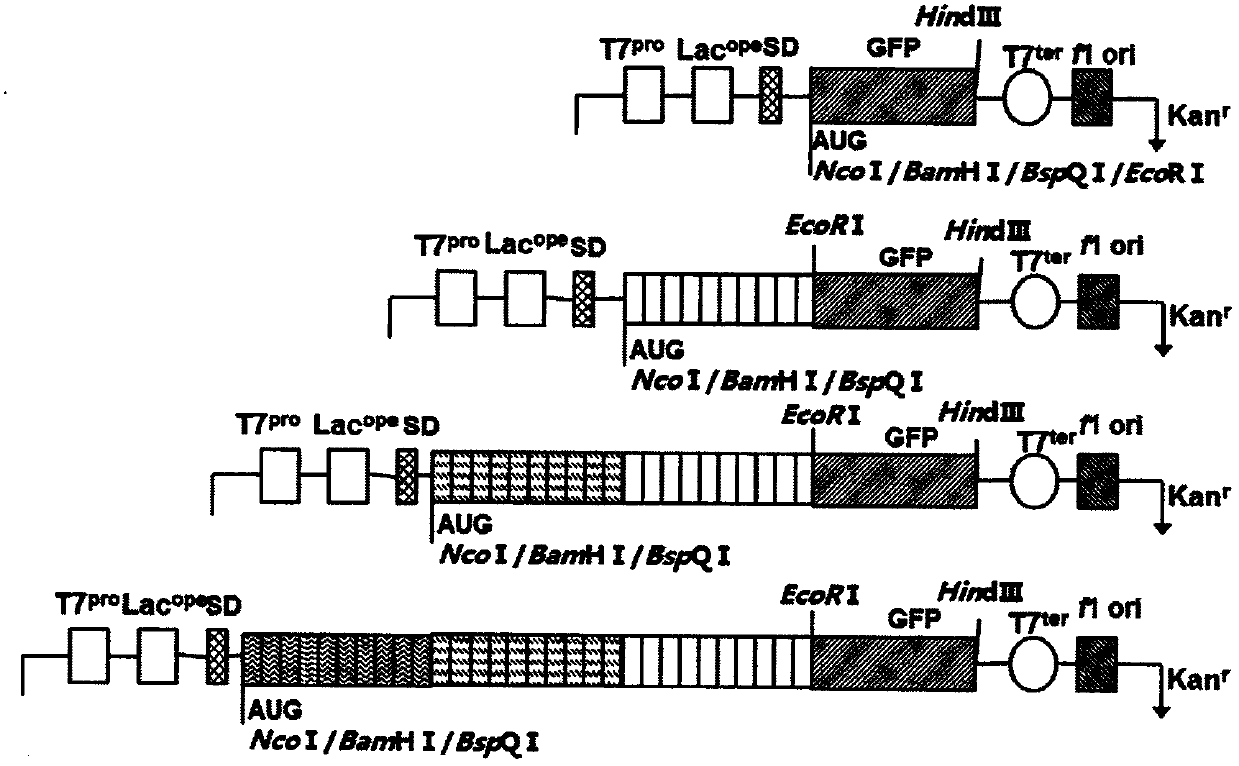
[0068] The following examples describe methods for constructing short sequence segments of 20 amino acids in length. As described in Example 1, Example 2 can also design this section from scratch. At the same time, the construction of a codon-optimized gene set encoding a sequence of 20 amino acids can also be described by taking a short sequence containing 10 amino acids as an example. In the first step, the pET28a vector was transformed to remove the BspQ I site on the vector framework region. The transformed vector was named DMT vector. The GFP gene (SEQ NO: 155) (with Nco I, BamH I, BspQ I, EcoR I at the 5' end and a HindIII restriction site at the 3' end) was cloned into the Nco I and HindIII sites of the DMT vector , to obtain the recombinant vector DMT-Nco I-BamH I-BspQ I-EcoR I-GFP-HindIII. The carrier DMT is digested with BspQ I, and a stuffer sequence can be inserted at the B...
Embodiment 3
[0072] Example 3: Construction of a short sequence segment of 18 amino acids in length
[0073] As described in Example 2 below, there are two ways to construct the PT18 sequence segment. First, as described in Example 1, the P18 sequence segment was directly designed and synthesized. Second, as for the screening method described in Example 2, the construction of a codon-optimized gene set encoding a sequence of 18 amino acids can be described by taking 9 amino acid sequences as an example. The DMT vector was constructed by the same method as in Example 2. Digestion of vector DMT with BspQ I allows insertion of a stuffer sequence at the BspQ I site. The polypeptide sequence of 18 amino acids is named P18. Its 18 amino acid sequences have [X]2, where X is a peptide segment containing 9 amino acids, and 6 types of amino acids are selected from glycine, alanine, serine, threonine, proline, and lysine. The 9-amino acid peptide segment is named sequence library PT06, and the se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com