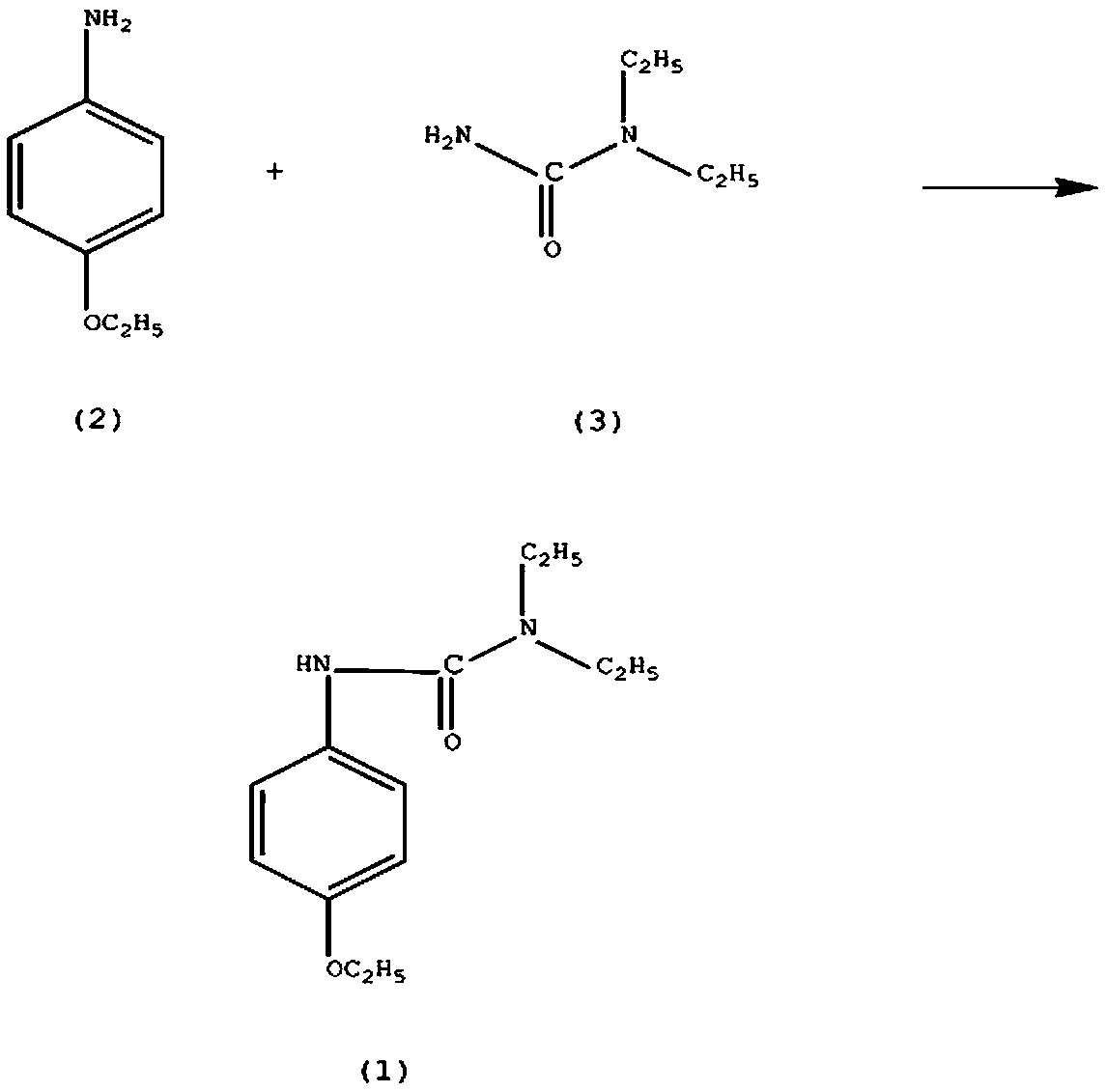
Synthesis method of celiprolol drug intermediate 3-(4-enthoxyphenyl)-1,1-diethylurea
A technology of ethoxyphenyl and diethylurea, which is applied in the field of synthesis of 3--1,1-diethylurea, a medicine intermediate of celilolol, can solve the problem that the vasodilation effect of isoproterenol cannot be reversed. and other problems, to achieve the effect of reducing intermediate links, reducing reaction temperature and reaction time, and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] In a reaction vessel equipped with a stirrer and a dropping funnel, add 0.22mol of p-aminophenethyl ether (2), 0.45mol of sodium bisulfite, and 80ml of tetrahydrofuran, control the stirring speed at 130rpm, control the solution temperature at 35°C, and add N , N-diethylaminocarboxamide (3) 0.25mol, the dropwise addition time is controlled at 4h, and the stirring reaction is continued for 30h, adding 300ml of a 10% sodium chloride solution with a mass fraction of 25% oxalic acid solution to adjust the pH of the solution to 4. Continue to react for 4 hours, solids are precipitated, lower the solution temperature to 10°C, filter, wash with potassium nitrate solution, dehydrate activated alumina, and recrystallize in hexane to obtain 3-(4-ethoxyphenyl)-1 , 1-diethylurea (1) 43.09g, yield 83%.
example 2
[0014] In a reaction vessel equipped with a stirrer and a dropping funnel, add 0.22mol of p-aminophenethyl ether (2), 0.46mol of sodium bisulfite, and 85ml of tetrahydrofuran, control the stirring speed at 150rpm, control the solution temperature at 37°C, and add N , N-diethylaminocarboxamide (3) 0.26mol, the dropwise addition time is controlled at 4h, and the stirring reaction is continued for 33h, adding 320ml of a 12% sodium chloride solution with a mass fraction of 27% oxalic acid solution to adjust the pH of the solution to 4. Continue to react for 4 hours, solids are precipitated, reduce the temperature of the solution to 13°C, filter, wash with sodium sulfate solution, dehydrate phosphorus pentoxide, and recrystallize in hexane to obtain 3-(4-ethoxyphenyl)- 1,1-diethylurea (1) 44.65g, yield 86%.
example 3
[0016] In a reaction vessel equipped with a stirrer and a dropping funnel, add 0.22mol of p-aminophenethyl ether (2), 0.47mol of sodium bisulfite, and 90ml of tetrahydrofuran, control the stirring speed at 160rpm, control the solution temperature at 40°C, and add N , N-diethylaminocarboxamide (3) 0.27mol, the dropping time is controlled at 5h, and the stirring reaction is continued for 35h, adding 350ml of 15% sodium chloride solution with a mass fraction of 30% oxalic acid solution to adjust the pH of the solution to 5. Continue to react for 5 hours, solids are precipitated, reduce the temperature of the solution to 15°C, filter, wash with potassium nitrate solution, dehydrate activated alumina, and recrystallize in hexane to obtain 3-(4-ethoxyphenyl)-1 , 1-diethylurea (1) 47.25g, yield 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com