Washing buffer solution for ion-exchange chromatography for preparation of FVIII (human coagulation factor VIII) and application of washing buffer solution
An ion exchange chromatography, washing buffer technology, applied in the direction of factor VII, coagulation/fibrinolysis factor, peptide preparation method, etc., can solve the problem that it is difficult to ensure that the visible foreign matter in the product is qualified, the preparation process of FVIII requires high, and the molecular weight of FVIII. It can achieve the effect of good industrial application prospects, good reconstitution performance and high activity yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 Preparation of human blood coagulation factor VIII of the present invention
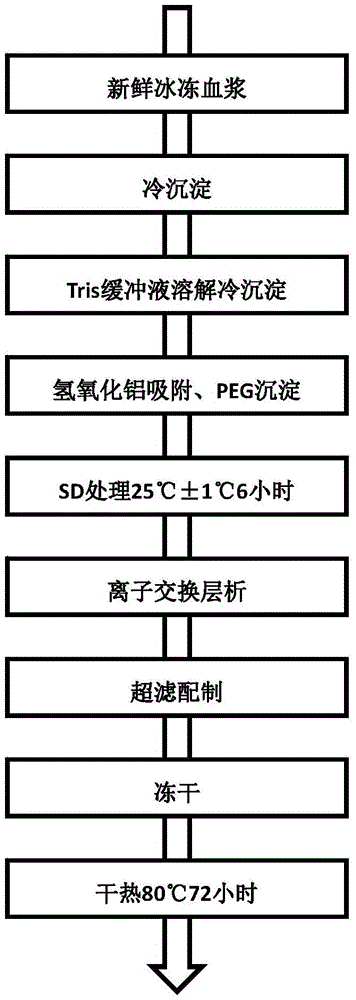
[0047] The preparation process of human coagulation factor VIII of the present invention is shown in figure 1 .
[0048] The preparation method is as follows:
[0049] Ⅰ. Pre-chromatographic treatment
[0050] (1) Using fresh frozen plasma as raw material, melt the plasma, centrifuge to prepare cryoprecipitate, dissolve the cryoprecipitate in 0.02M Tris buffer, precipitate with 30% polyethylene glycol, centrifuge after adsorption by aluminum hydroxide, and obtain the supernatant;
[0051] (2) After the supernatant was clarified and filtered at 0.45 μm, Tween-80 (polysorbate 80) and tributyl phosphate were added to make the final concentrations 1% and 0.3%, respectively, and treated at 25°C±1°C for 6 hours, and the process was completed. For the first virus inactivation (that is, SD virus inactivation), the sample must be loaded on the column.
[0052] Ⅱ. Ion exchange chromatog...
Embodiment 2
[0062] Embodiment 2 Preparation of human blood coagulation factor VIII of the present invention
[0063] The preparation method is as follows:
[0064] Ⅰ. Pre-chromatographic treatment
[0065] With embodiment 1.
[0066] Ⅱ. Ion exchange chromatography
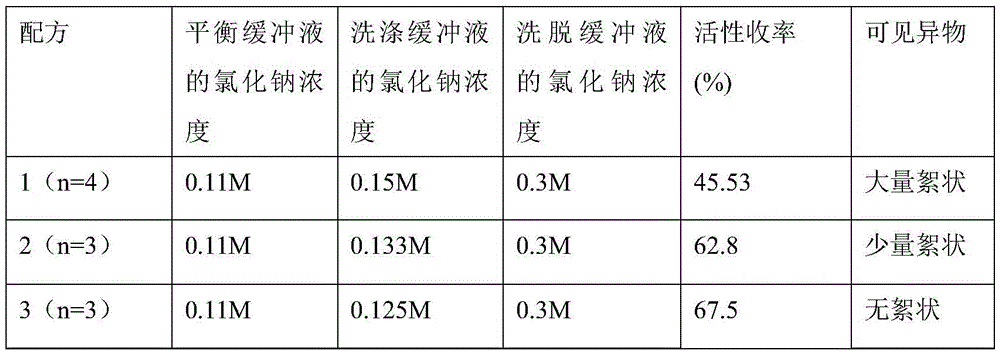
[0067] (1) Prepare buffer
[0068] The balance buffer includes the following components: 0.01M sodium citrate, 0.001M calcium chloride, 0.121M glycine, 0.016M lysine hydrochloride, 0.06M sodium chloride; adjust pH to 6.5;
[0069] The washing buffer includes the following components: 0.01M sodium citrate, 0.001M calcium chloride, 0.121M glycine, 0.016M lysine hydrochloride, 0.121M sodium chloride; adjust pH to 6.5;
[0070] The elution buffer includes the following components: 0.01M sodium citrate, 0.001M calcium chloride, 0.121M glycine, 0.016M lysine hydrochloride, 0.25M sodium chloride; adjust pH to 6.5;
[0071] (2) Use ToyopearlDEAE650M as the gel for the chromatographic column filler; put the sample to be loaded on ...
Embodiment 3
[0075] Embodiment 3 Preparation of human blood coagulation factor VIII of the present invention
[0076] The preparation method is as follows:
[0077] Ⅰ. Pre-chromatographic treatment
[0078] With embodiment 1.
[0079] Ⅱ. Ion exchange chromatography
[0080] (1) Prepare buffer
[0081] Equilibrium buffer includes the following components: 0.01M sodium citrate, 0.001M calcium chloride, 0.121M glycine, 0.016M lysine hydrochloride, 0.16M sodium chloride; adjust pH to 7.5;
[0082] The washing buffer includes the following components: 0.01M sodium citrate, 0.001M calcium chloride, 0.121M glycine, 0.016M lysine hydrochloride, 0.129M sodium chloride; adjust pH to 7.5;
[0083] The elution buffer includes the following components: 0.01M sodium citrate, 0.001M calcium chloride, 0.121M glycine, 0.016M lysine hydrochloride, 0.35M sodium chloride; adjust pH to 7.5;
[0084] (2) Use ToyopearlDEAE650M as the gel for the chromatographic column filler; put the sample to be loaded on ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
| Specific activity | aaaaa | aaaaa |
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com