Cyanide-free electroplating baths for white bronze based on copper (I) ions
An electroplating bath and ion technology, applied in the field of cyanide-free electroplating baths, can solve problems such as increasing costs and reducing overall process efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] Copper / tin / silver ternary white bronze
[0051] Prepare the following aqueous acid white bronze plating bath:
[0052] Table 1
[0053] compound
Concentration(g / L)
Copper(I) ion in the form of copper oxide
30
Tin(II) ion in the form of tin methanesulfonate
12
Silver(I) ion in the form of silver methanesulfonate
5
1-(2-Dimethylamino-ethyl)-5-mercapto-1,2,3,4-tetrazole
96
3,6-Dithia-1,8-octanediol
75
Methanesulfonic acid (70%)
150g / L
Antimony in the form of potassium antimony tartrate
0.16
Nonionic Phenol Ethoxylates 1
0.8
hydroquinone monosulfonic acid
1g / L
[0054] 1 Adeka Tol PC-8: Non-ionic surfactant, commercially available from Adeka Corporation.
[0055] The pH of the bath was less than 1 as measured using a KNICK Instruments conventional laboratory pH meter. The molar masses of tetrazole compound, 3,6-dithia-1,8-octanediol and copper(I) ion a...
example 2
[0059] Copper / tin binary white bronze
[0060] Prepare the following aqueous acid white bronze plating bath:
[0061] Table 2
[0062] compound
Concentration(g / L)
Copper(I) ion in the form of copper oxide
14
Tin(II) ion in the form of tin methanesulfonate
8
1-(2-Dimethylamino-ethyl)-5-mercapto-1,2,3,4-tetrazole
42
thiodiethanol
80
hydroquinone monosulfonic acid
1.6g / L
Methanesulfonic acid (70%)
90g / L
bismuth methanesulfonate
0.02
1,10-Phenanthroline Monohydrate
0.01
Nonionic Phenol Ethoxylates 2
0.8
[0063] 2 Adico TolPC-8: non-ionic surfactant, available from Addico.
[0064] The pH of the bath solution is less than 1 as measured with a Konik Instruments routine laboratory pH meter. The molar ratio of tetrazole to copper(I) ions in the bath was 1.1:1, and the molar ratio of tetrazole to thiodiethanol was 0.4:1.
[0065] Use RONACLEAN on a brass panel w...
example 3
[0068] Tetrazole / 3,6-dithia-1,8-octanediol molar ratio in copper / tin / silver electroplating baths
[0069] White bronze copper / tin / silver alloy electroplating baths were prepared as described in Example 1 except that the amount of 3,6-dithia-1,8-octanediol was varied as shown in Table 3 below. The molar ratio of 1-(2-dimethylamino-ethyl)-5-mercapto-1,2,3,4-tetrazole to 3,6-dithia-1,8-octanediol is listed in Table 3 shown.
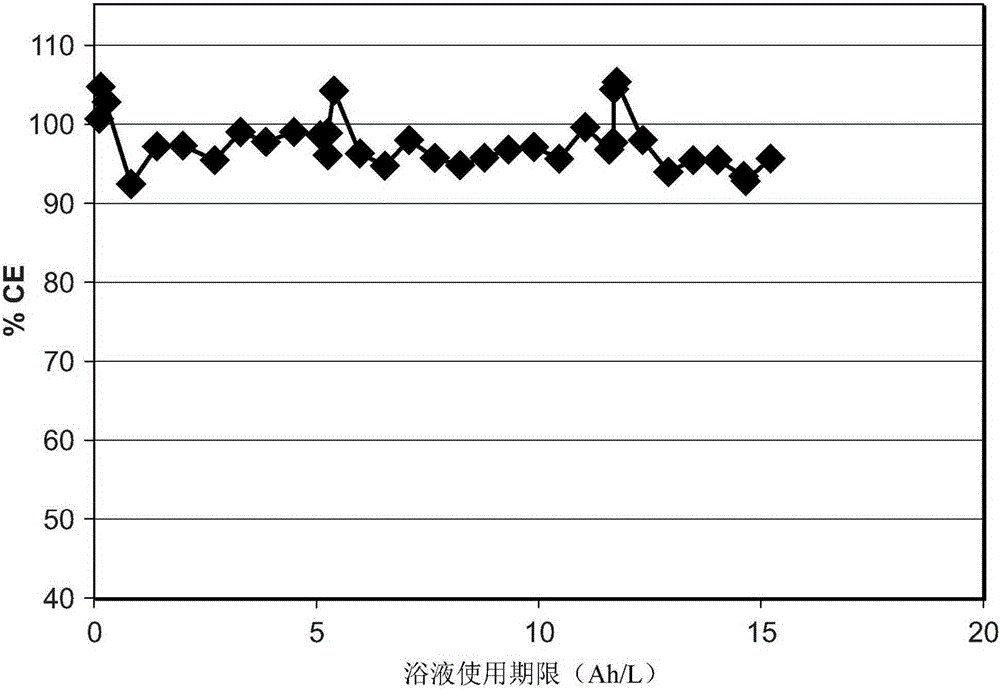
[0070] Brass panels measuring 10 x 7.5 x 0.025 cm were degreased and activated as described in Example 1 above. Each panel was then placed in a separate Hull cell containing a 250 mL white bronze bath. The pH of the bath is less than 1. Platinized titanium or bronze electrodes are used as anode material. The working bath temperature is in the range of 35°C to 45°C. The panels were electroplated with a white bronze bath at 1A for 3 minutes. All baths appeared to be stable throughout plating.
[0071] After plating, the panels were removed from the Hull...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com