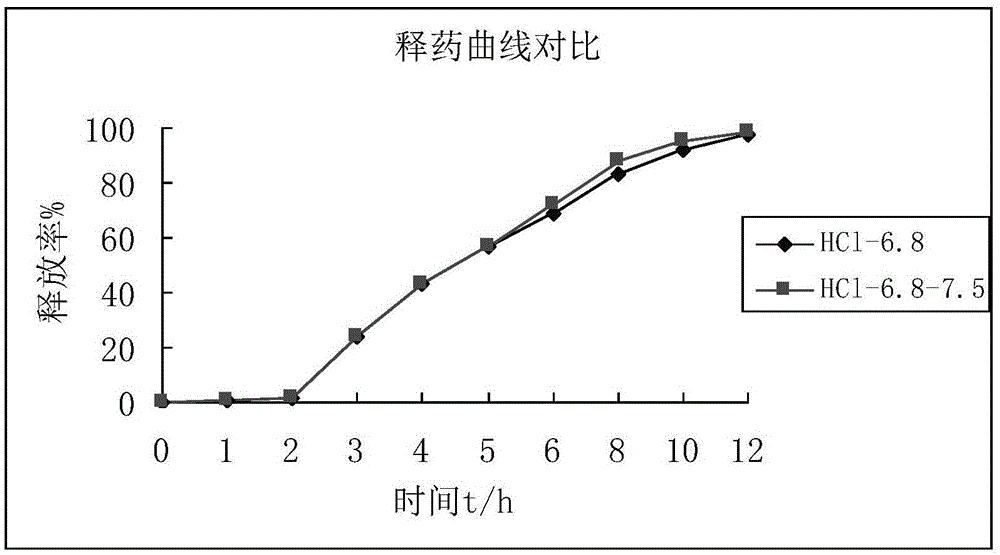
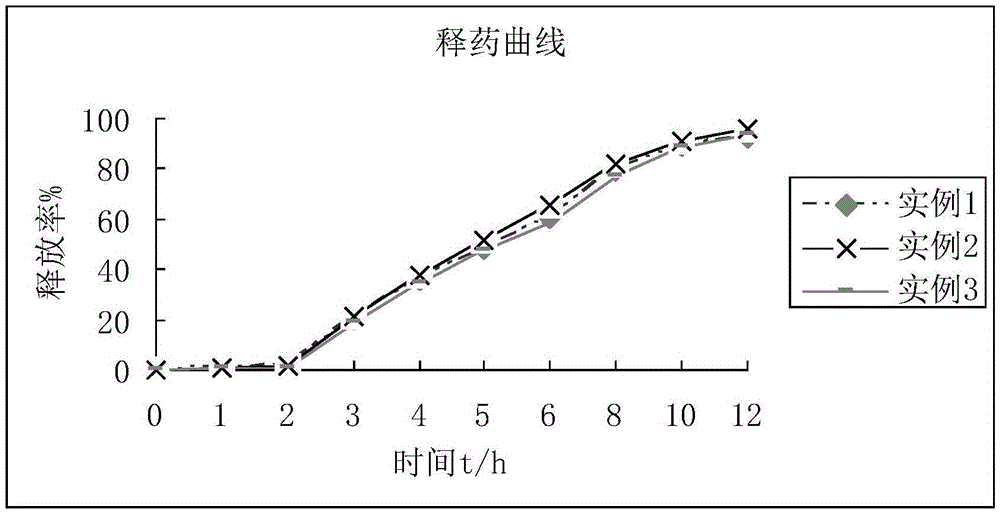
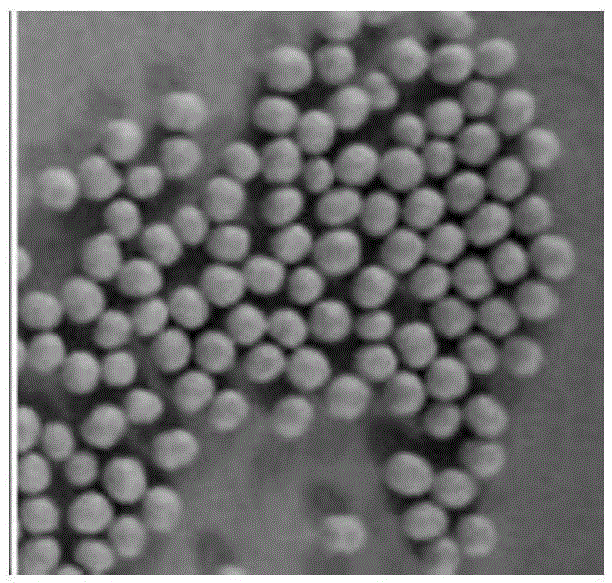
Mesalazine sustained-release pellets, preparation method thereof and mesalazine sustained-release capsule
A technology of sustained-release pellets and mesalazine, applied in the field of mesalazine sustained-release pellets and its preparation, and mesalazine capsules, which can solve the problems that the drug release effect needs to be improved, and achieve favorable treatment and high drug loading , reduce the effect of stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] Correspondingly, the present invention provides a method for preparing mesalazine sustained-release pellets, comprising: mixing 5-aminosalicylic acid, microcrystalline cellulose and a binder, and extruding and spheronizing to obtain a pellet containing a core ;
[0056] Using a material including ethyl cellulose, wrapping a sustained-release coating layer on the core of the drug-containing pill;
[0057] The enteric coating layer is wrapped on the sustained release coating layer by adopting the enteric material and processing aid to obtain the mesalazine sustained release pellets; the enteric material is a copolymer of methacrylic acid and ethyl acrylate.
[0058] The embodiment of the present invention adopts an extrusion spheronization process, adding water to 5-aminosalicylic acid and auxiliary materials including microcrystalline cellulose and a binder, and drying them to make a drug-containing pellet core.
[0059] Wherein, the contents of the microcrystalline cel...
Embodiment 1
[0075] Prescription with pill core (1000 capsules):
[0076]
[0077] Adhesive preparation: Weigh 119g of pure water and 21g of PVPk30, add 21g of PVPk30 to 119g of pure water under constant stirring at 500rpm, and stir for 20min to obtain a 15% PVPk30 aqueous solution.
[0078] Ball core preparation: Take the prescribed amount of mesalazine and microcrystalline cellulose and mix them evenly after sieving respectively, use the prepared 15% PVPk30 aqueous solution as a binder to prepare a soft material, and place the soft material in an extrusion spheronizer The pellets were prepared with an extrusion speed of 25 rpm, a spheronization speed of 900 rpm, and a spheronization time of 3 minutes; the pellets were dried in an oven at 40°C, and sieved to 20-30 meshes to obtain coating cores.
[0079] Sustained-release layer coating liquid prescription:
[0080] Su Lisi (Shanghai Colorcon Coating Technology Co., Ltd.) 150g
[0081] Pure water 100g
[0082] Preparation of sustaine...
Embodiment 2
[0092] Prescription with pill core (1000 capsules):
[0093]
[0094] Adhesive preparation: Weigh 127g of pure water and 11g of HPMCE15, add 11g of HPMCE15 into 127g of pure water under constant stirring at 500rpm, stir for 20min, and leave overnight to obtain an 8% HPMCE15 aqueous solution.
[0095] Ball core preparation: Take the prescribed amount of mesalazine and microcrystalline cellulose, sieve them and mix them evenly, use the prepared 8% HPMCE15 aqueous solution as a binder to prepare a soft material, and place the soft material in an extrusion spheronizer Micropellets were prepared; the extrusion speed was 25 rpm, the spheronization speed was 900 rpm, and the spheronization time was 3 min. The pellets were dried in an oven at 40°C, and sieved to 20-30 meshes to obtain pellet cores for coating.
[0096] Sustained-release layer coating liquid prescription:
[0097] Su Lisi (with embodiment 45g1)
[0098] Pure water 30g
[0099] Preparation of sustained-release co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com