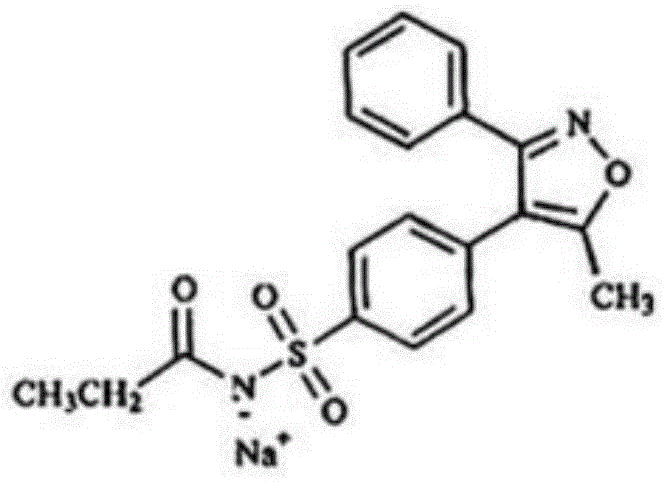
Parecoxib sodium pharmaceutical composition for injection and preparation method thereof
A technology of parecoxib sodium and parecoxib meter, which is applied in the field of parecoxib sodium pharmaceutical composition for injection and its preparation, and can solve the problems of small dosage of the main drug, high water content, and high requirements for production equipment , to achieve the effect of reducing dosage, meeting safety requirements and avoiding adverse events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Parecoxib Sodium Preparation Method 1
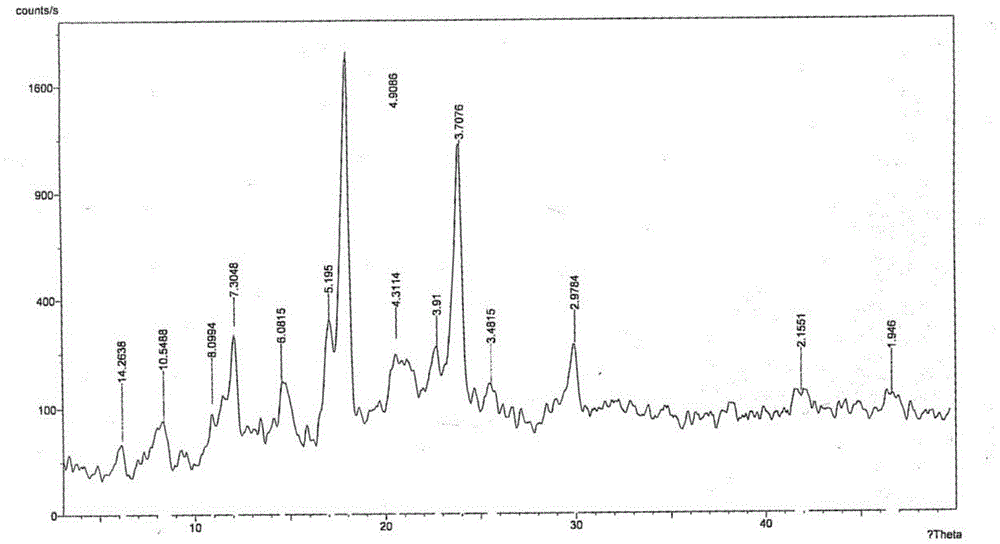
[0062] Add 100g of the crude product of parecoxib sodium into 100ml of acetone, stir mechanically, heat up to reflux, add 1000ml of acetonitrile dropwise, keep stirring for 1 hour, then cool down to room temperature, filter, rinse the filter cake with acetonitrile, and dry under reduced pressure to obtain 94.4 g Parecoxib sodium was crystallized, and the yield was 94.4%. Related substances adopt HPLC method, and the total impurity is 0.02%; use Cu-ka ray to carry out X-ray powder measurement, with the following description: figure 1 X-ray powder diffraction pattern shown.
Embodiment 2
[0063] Embodiment 2: Parecoxib sodium preparation method 2
[0064] Add 100g of crude parecoxib sodium to 100ml of ethanol, stir mechanically, heat up to reflux, add 1000ml of methyl tert-butyl ether dropwise, keep stirring for 1 hour, cool down to room temperature, filter, filter cake with methyl tert-butyl Rinse with ether and dry under reduced pressure to obtain 94.8 g of parecoxib sodium crystals with a yield of 94.8%. Related substances adopt HPLC method, and the total impurity is 0.02%; use Cu-ka ray to carry out X-ray powder measurement, its X-ray powder diffraction pattern and attached figure 1 unanimous.
Embodiment 3
[0066] Embodiment 3: Parecoxib sodium for injection (in 1000 bottles, unit: g)
[0067] Parecoxib Sodium
[0068] Preparation process: pour 50% of the prepared water for injection into the liquid preparation tank, cool down to make the temperature lower than 40°C, add the prescribed amount of parecoxib sodium and anhydrous disodium hydrogen phosphate, and stir until dissolved; Adjust the pH to 8.0 with sodium hydroxide or phosphoric acid solution, add 0.05% (W / V) medicinal charcoal, stir and adsorb for 20 minutes, filter with a 0.22 μm PES filter, and then add water for injection to the full amount; fill in 1ml per bottle Place in a vial; freeze-dry in a freeze-drying box, drop the plate layer from room temperature to -40°C for 30 minutes, and maintain it for 4 hours to completely freeze the sample. Turn on the condenser, and when the temperature of the condenser drops below -45°C , turn on the vacuum pump to evacuate, the pre-vacuum is lower than 0.2mbar, and start ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com