Preparation method and application of chondroitin sulfate ABC enzyme fusion protein
A chondroitin sulfate, fusion protein technology, applied in the directions of lyase, oxidoreductase, recombinant DNA technology, etc., can solve the problems of cumbersome preparation process, cumbersome extraction steps, limited research on heterologous recombinant expression, etc., and achieves simplified purification procedures, Generate low-quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
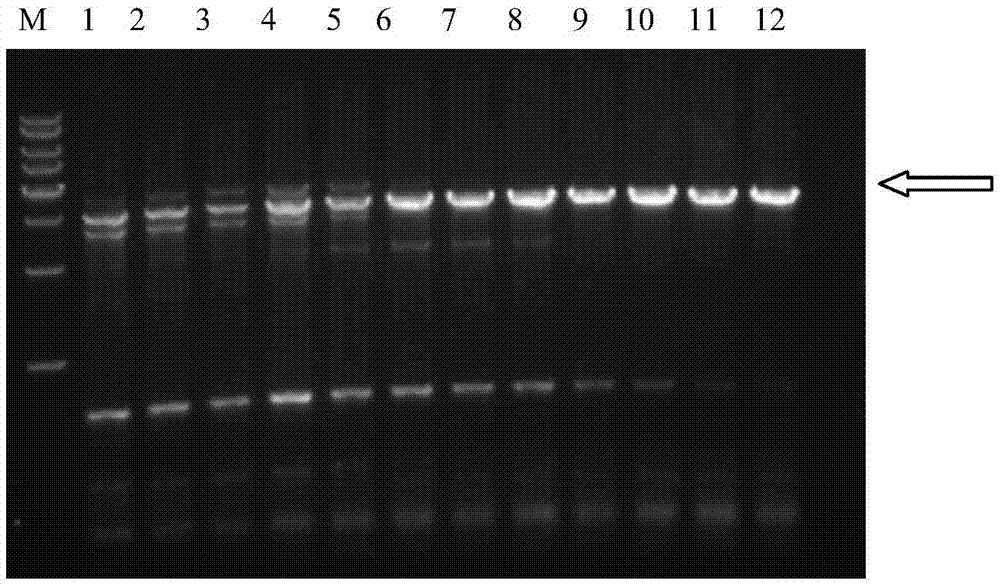
[0093] Embodiment 1, the expression of chondroitin sulfate enzyme ABC fusion protein (GAPDH-ChSaseABCI) Express
[0094] 1. Cloning of Proteus vulgaris chondroitin sulfate ABC enzyme coding sequence and glyceraldehyde 3-phosphate dehydrogenase DNA sequence without signal peptide
[0095] The specific process of constructing the expression vector pMAL-c2x-ChSaseABCI is as follows:
[0096] 1.1 Design and synthesis of primers
[0097] The DNA sequences of Proteus vulgaris chondroitin sulfate ABC enzyme and glyceraldehyde 3-phosphate dehydrogenase were obtained through Genbank query, and the upstream and downstream primers used were:
[0098] Upstream primer P1: 5'-CG GGATCC ATGGCCACCAGCAATCCTGCATT-3' (SEQ ID NO: 5) (the underlined base is the restriction site of BamHI),
[0099] Downstream primer P2: 5'-AA CTGCAG TTATCAAGGGAGTGGCGAGAGTTTG-3' (SEQ ID NO: 6) (the underlined base is the PstI restriction site), after amplification, the BamHI and PstI restriction sites were...
Embodiment 2
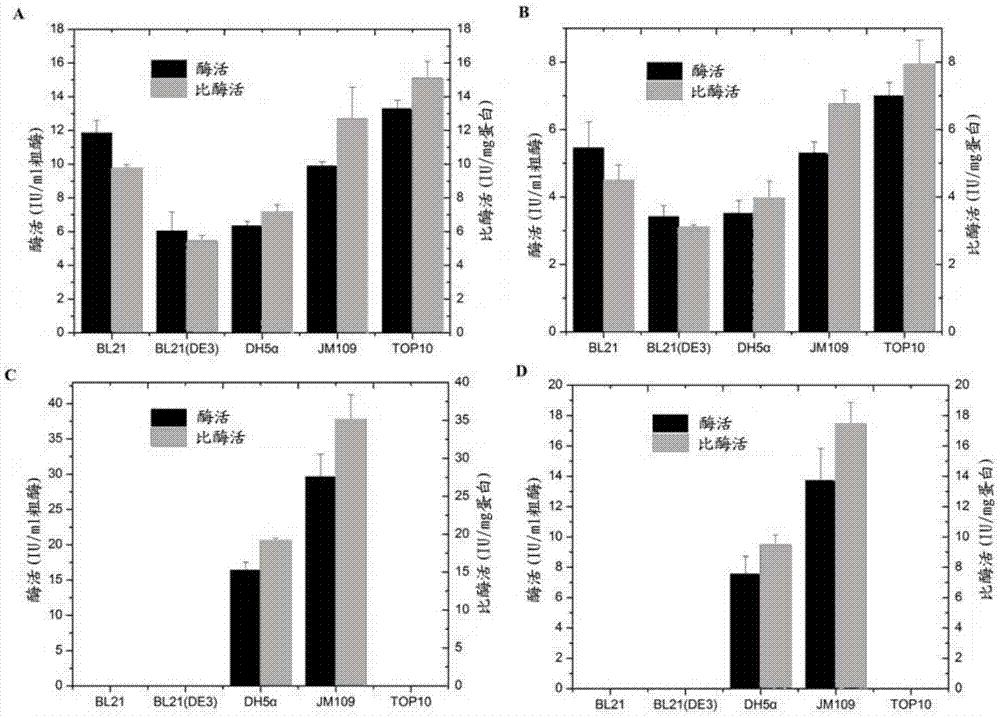
[0127] Embodiment 2, purify chondroitin sulfate ABC enzyme fusion protein by Ni column GAPDH-ChSaseABCI
[0128] In this example, a Ni column is used to realize affinity adsorption to realize one-step separation.
[0129] The specific steps of affinity separation are as follows: 50 mL of the bacterial cells whose final concentration was 0.5 mMIPTG induced expression of 24 were centrifuged at 6000 rpm for 10 minutes; at the same time, the bacterial cells without induced expression were set as a control. Then proceed with the following two options:
[0130] Wash twice with column equilibration solution Columnbuffer (20mM Tris-HCl, 200mMNaCl, pH7.4), resuspend in 30mL Columnbuffer, carry out sonication (output power is 300W, each sonication 5 seconds and interval 6 seconds, the total processing time is 15 minute).
[0131] After centrifugation, the supernatant was passed through a 1 mL pre-equilibrated Ni affinity separation column at 1 mL / min, eluted by the eluent and coll...
Embodiment 3
[0144] Embodiment 3, utilize the chondroitin sulfate ABC enzyme that embodiment 2 prepares to produce low molecular weight sulfuric acid Chondroitin A, B or C
[0145] The chondroitin sulfate ABC enzyme with a purity of 90% was obtained in Example 2. This embodiment uses the purified chondroitin sulfate ABC enzyme to produce low molecular weight chondroitin sulfate A, B or C.
[0146] Concrete production method is as follows: preparation concentration is respectively the chondroitin sulfate A of 100g / L, the solution 1L of B and C, and chondroitin sulfate A, B and C molecular weight are 50kDa, derive from bovine cartilage tissue (purchased from Nanjing Odufoni Biotechnology Co., Ltd.), as a reaction substrate, add 10-20ml of purified chondroitinase (13922IU / L). The reaction temperature is 30-55°C, and 15 mL of new enzyme solution is added every 1 hour, and samples are taken to measure the reaction product at the same time, and the reaction time is 5-10 hours.
[0147] Afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com