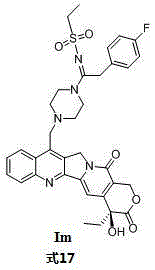
Camptothecin sulfonyl amidine compound and preparation method and application thereof
A base sulfonamide compound technology, applied in the field of camptothecin sulfonamide compounds and their preparation and application, can solve the problems of non-direct parenteral administration to human body, bone marrow suppression, vomiting and diarrhea, severe side effects, etc. problem, to achieve the effect of excellent application prospect, novel structure and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
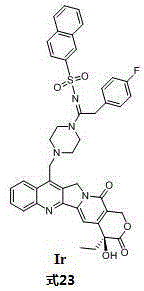
[0018] Embodiment 1: the synthesis of target compound Ia
[0019]
[0020] Synthesis of raw material 7-benzylpiperazine-camptothecin: Take 2.5 grams of camptothecin in a round bottom flask, add 60 mL of 75% sulfuric acid solution to stir and dissolve, then add 3.125 grams of sulfuric acid heptahydrate under ice bath conditions Ferrous, stirred for 5min, still under the condition of ice bath, dropwise added 40mL of 40% chloroacetaldehyde solution with the constant pressure funnel, stirred for 5min after the dropwise addition, still under the condition of ice bath, added 12.5mL with the constant pressure funnel, hydrogen peroxide. The ice bath was removed after half an hour of reaction. After reacting for 10 hours at room temperature, put the reaction in an ice bath, then add an appropriate amount of ice water, a large amount of yellow solids precipitated, filter with suction, rinse the filter cake with ice water several times, and then rinse the filter cake with a solution ...
Embodiment 2
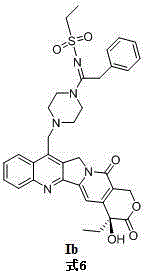
[0027] Embodiment 2: the synthesis of target compound Ib
[0028]
[0029] Same as Example 1, only ethylsulfonyl azide is used instead of methylsulfonyl azide. The detection data of the product obtained from the reaction are as follows: yield: 67%; melting point: 233-238 o C; 1 HNMR (DMSO -d 6 ,400MHz) δ :8.40(d,1H, J =8Hz,C9-H),8.15(d,1H, J =8Hz,C12-H),7.84(t,1H, J =8Hz,C10-H),7.70(t,1H,J =8Hz,C11-H),7.36-7.31(m,3H,Ar-H,C14-H),7.28-7.24(m,3H,Ar-H),6.54(s,1H,C20-OH),5.43 (s,2H,C17-H),5.31(s,2H,C5-H),4.39(s,2H,- CH 2 -Ar),4.05(s,2H,- CH 2 -piperazine), 3.67 (s, 2H, piperazine-H), 3.31 (s, 2H, piperazine-H), 3.00-2.92 (m, 2H, -SO 2 - CH 2 -),2.54(s,2H,piperazine-H),2.28(s,2H,piperazine-H),1.92-1.80(m,2H,C19-H),1.26-1.14(m,3H,-SO 2 -CH 2 - CH 3 ),0.88(t,3H, J =8Hz,C18-H); MS-ESIm / z:678.3[M+Na] + ..
Embodiment 3
[0030] Embodiment 3: the synthesis of target compound Ic
[0031]
[0032] Same as Example 1, only phenylsulfonyl azide is used instead of methylsulfonyl azide. The detection data of the product obtained from the reaction are as follows: productive rate: 65%; melting point: 209-212 o C; 1 HNMR (DMSO -d 6 ,400MHz) δ :8.39(d,1H, J =8Hz,C9-H),8.15(d,1H, J =8Hz,C12-H),7.86-7.79(m,3H,C10-H,Ar-H),7.69(t,1H, J =8Hz,C11-H),7.57-7.47(m,3H,Ar-H),7.33-7.21(m,6H,C14-H,Ar-H),6.53(s,1H,C20-OH),5.43 (s,2H,C17-H),5.30(s,2H,C5-H),4.44(s,2H,- CH 2 -Ar),4.04(s,2H,- CH 2 -piperazine), 3.67(s,2H,piperazine-H),3.29(s,2H,piperazine-H),2.54(s,2H,piperazine-H),2.29(s,2H,piperazine-H),1.90- 1.81(m,2H,C19-H),0.87(t,3H, J =8Hz,C18-H); MS-ESIm / z:726.3[M+Na] + ..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com