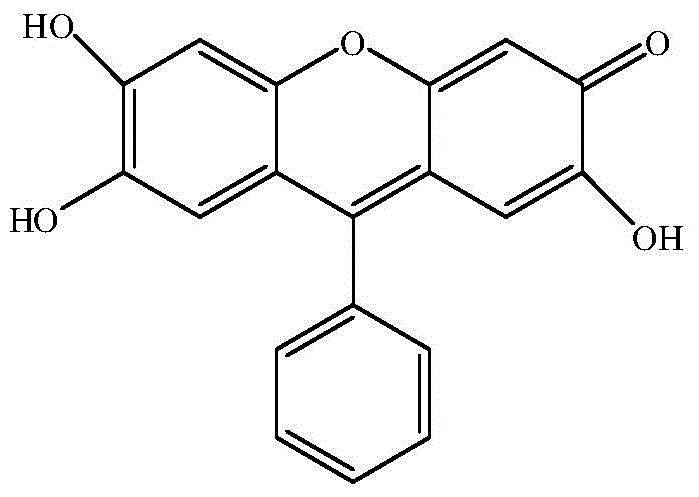
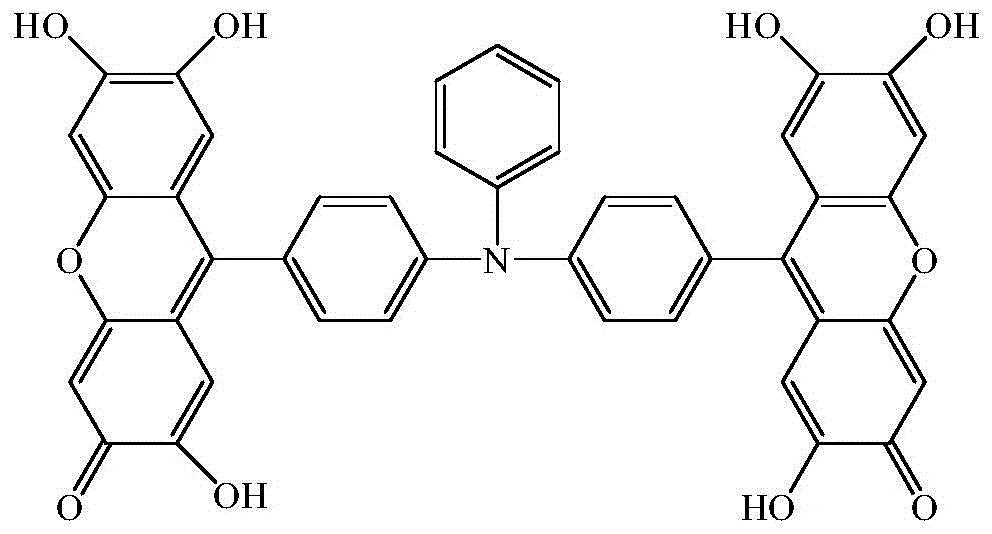
9, 9'-(N-phenyl-N, N-4, 4'-diphenyl) double fluorescein reagent and preparation method and application thereof
A dual-fluorescein and diphenyl technology, which is applied in the directions of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of unsatisfactory sensitivity and selectivity of reagents for detecting heavy metal ions, and achieve a simple preparation method and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
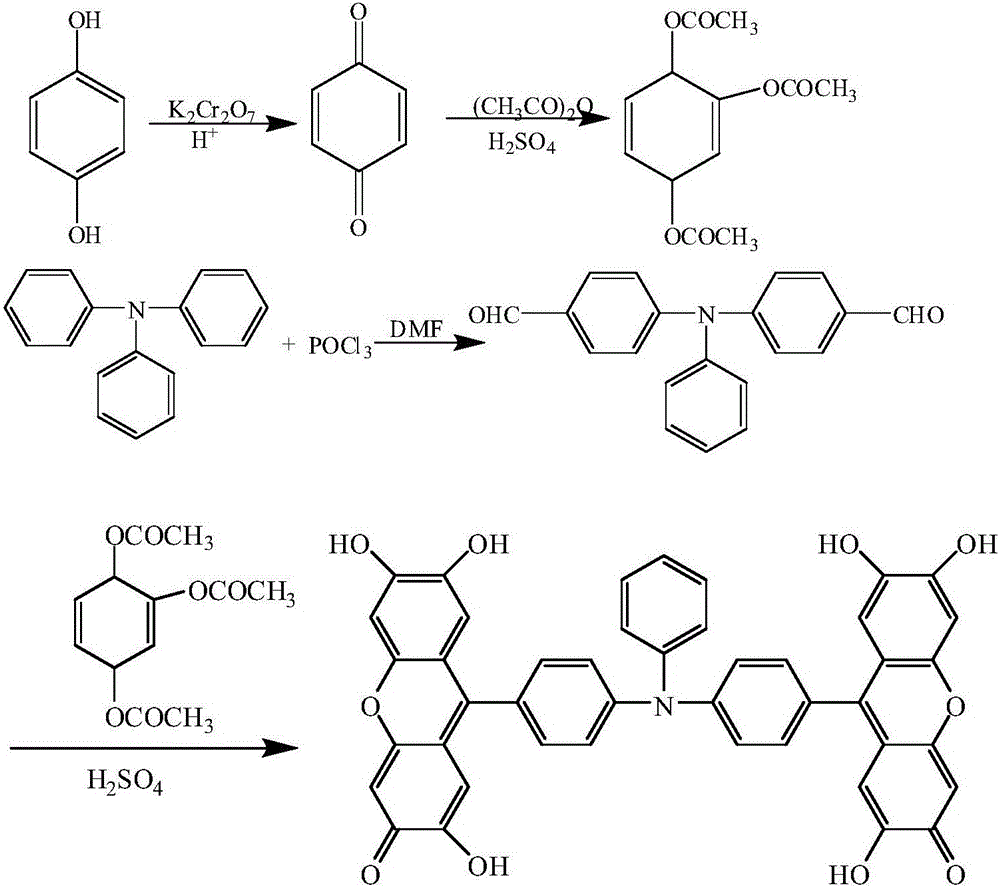
[0032] Example 1: A preparation method of 9,9'-(N-phenyl-N,N-4,4'-diphenyl)bisfluorone
[0033] The reaction formula is as follows:
[0034]
[0035] A method for preparing 9,9'-(N-phenyl-N,N-4,4'-diphenyl)bisfluorone, comprising the following steps:
[0036] (1) Synthesis of p-benzoquinone
[0037] Dissolve 20.0g of hydroquinone in a 1000mL long-necked flask filled with 333mL of 50°C hot water, and then slowly add 20mL of concentrated H 2 SO 4 , use crushed ice to strictly control the reaction temperature at 20-30°C, then slowly add 266.6mL K 2 Cr 2 o 7 solution (contains K 2 Cr 2 o 7 32.79g). After the reaction, the crude product of p-benzoquinone was obtained by suction filtration, washed with cold water and dried to obtain 9.5 g of yellow p-benzoquinone with a yield of 48.3%.
[0038] (2) Synthesis of trimerol triacetate
[0039]In a 100 mL round bottom flask, add 40.0 mL of acetic anhydride and 2.0 mL of concentrated sulfuric acid in sequence, and add 9.5 g...
Embodiment 2
[0049] Example 2: 9,9'-(N-phenyl-N,N-4,4'-diphenyl)bifluorone reagent spectrophotometry on Zn 2+ Analysis and detection of ions
[0050] In a 25mL volumetric flask, accurately pipette no more than 15μg of Zn 2+ , followed by adding 2.0mL of borax-NaOH buffer solution with pH 10.6, 1.5mL of CPB (cetylpyridinium bromide)-OP microemulsion (CPB:OP:n-butanol:n-heptane:H 2 The mass ratio of O is 1:5:3.5:0.8:89), 2.0mL 0.3g / L 9,9'-NNBPBF ethanol solution, then dilute to the mark with twice distilled water, shake well and let it stand for 15min, then use 1cm ratio The color dish is at 620nm, and the absorbance of the system is measured with the reagent blank as a reference.
[0051] According to the literature [Analytical Chemistry, Wei Qin et al., 2004, Vol. 32, No. 11, No. 1509-1512], accurately weigh 5 g of the sample, place it in a muffle furnace and incinerate at 600 °C for 4 h, add a small amount of hydrochloric acid (1+1) to dissolve the residue, and then add 4 mL Nitric aci...
Embodiment 3
[0056] Example 3: 9,9'-(N-phenyl-N,N-4,4'-diphenyl)bifluorone reagent fluorescence quenching method on Cu 2+ Analysis and detection of ions
[0057] In a 25mL volumetric flask, add no more than 5μg of Cu 2+ , add 2.0mL 1.0×10 -4 mol / L 9,9'-NNBPBF ethanol solution, 3.0 mL pH=8.0 triethanolamine-hydrochloric acid buffer solution, 3.0 mL absolute ethanol, 4.0 mL 0.5% cetyltrimethylammonium bromide (CTMAB) Solution, then diluted with water to the mark, shake well. Prepare the reagent blank in the same way. Leave it for 10min. on a spectrofluorometer at λ ex =556nm,λ em = Measure the fluorescence intensity F, F of the test solution and the blank at 596nm 0 , calculate the fluorescence quenching value ΔF=F 0 -F.
[0058] According to the literature [Analytical Chemistry, 2004,32(6):838], accurately weigh 1g of clean hair sample in a small beaker, add 10mL HNO 3 , digested by heating at low temperature until the brown gas escapes, and the solution is light yellow, add 1mL H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com