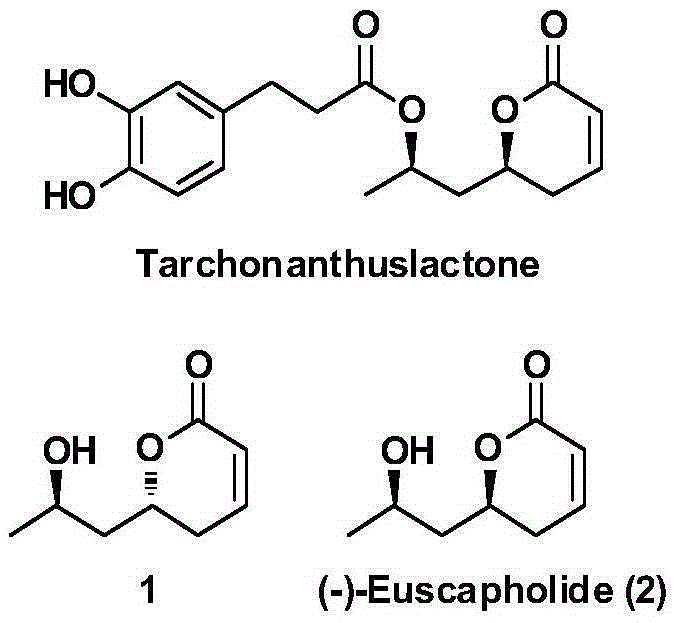
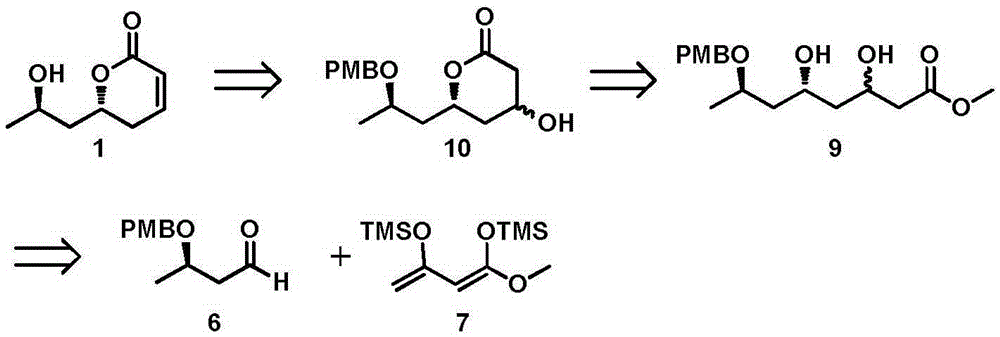
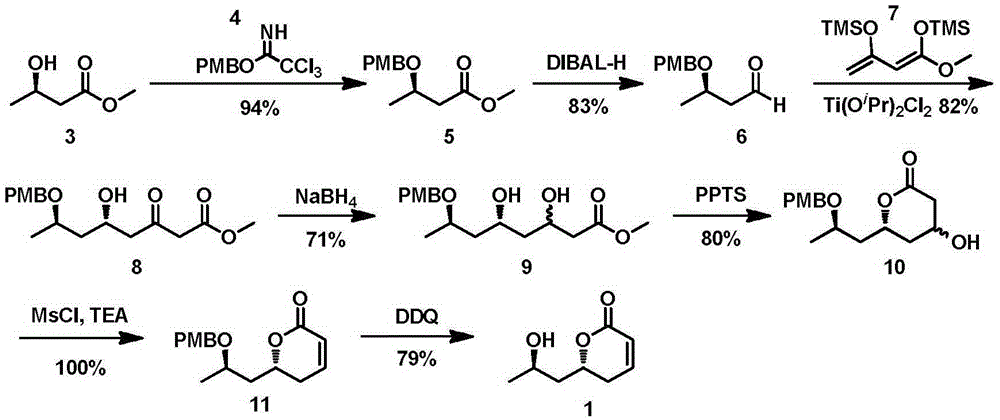
New method for asymmetrically synthesizing natural product (-)-Euscapholide isomer
A technology of natural products and synthetic methods, applied in the fields of organic chemistry, bulk chemical production, organic chemistry, etc., can solve the problems of complicated post-processing, long synthetic routes, expensive reagents, etc., and achieves easy availability of raw materials, less side reactions, The effect of reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1, formula 5 compounds
[0035] Before synthetic formula 5, at first synthetic formula 4 compound, carry out according to following process:
[0036] At 0°C and under the protection of nitrogen, sodium hydride powder (161.6 mg, 2 mmol) was slowly added to a solution of p-methoxybenzyl alcohol (2 mL, 8 mmol) in ether (6 mL), then warmed to room temperature, and The reaction was stirred for 30 minutes. Then the reaction mixture was cooled to 0°C again, and trichloroacetonitrile (1.6 mL, 8 mmol) was added at the same time, then the reaction mixture was warmed to room temperature, and after stirring for 2 hours, saturated sodium bicarbonate solution was added to quench the reaction. The obtained mixed system was extracted with dichloromethane (3×20mL) and the organic phases were combined, washed with saturated brine and dried over anhydrous sodium sulfate, then concentrated under reduced pressure to remove the organic solvent to obtain an orange...
Embodiment 2
[0038] The synthesis of embodiment 2, formula 6 compound
[0039] At -78°C, diisobutylaluminum hydride solution (22.44mL, 22.44mmol, 1M n-hexane solution) was slowly added dropwise to a solution of compound of formula 5 (4.45g, 18.70mmol) in dichloromethane (50mL), After completion of the dropwise addition, the reaction was carried out at this temperature for 2 hours. After the reaction was over, methanol (1 mL) was added to the reaction system at -78°C to quench the reaction, then warmed up to room temperature, and 10 mL of water was added to separate the layers. The aqueous phase was then extracted with dichloromethane (2×50 mL), and the combined The obtained organic phase was washed with saturated brine, and the obtained organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the organic solvent, and the obtained crude product was subjected to flash column chromatography (ethyl acetate: petroleum Ether=1:15) separated and purifi...
Embodiment 3
[0040] Synthesis of embodiment 3, formula 8 compound
[0041] Before synthesizing the compound of formula 8, it is necessary to make enol silyl ether 7 by itself, and its preparation process is as follows:
[0042] Add triethylamine (43.5mL, 314.40mmol) into a solution of methyl acetoacetate (12.0g, 103.34mmol) in n-hexane (260mL), then slowly add trimethylchloromethane (18.2mL, 141.73mmol), after reacting at room temperature for 18 hours, filter out the solid in the reaction system and concentrate, and the concentrated solution is distilled under reduced pressure to obtain 3-trimethylsilyloxy-2-butenoic acid methyl ester (9.37g, 61%) for the following reaction. 1 HNMR (400MHz, CDCl 3 )δ:5.12(s,1H),3.65(s,3H),2.26(s,3H),0.26(s,9H).
[0043] Dissolve 3-trimethylsilyloxy-2-butenoic acid methyl ester (9g, 47.80mmol) in anhydrous tetrahydrofuran (40mL), cool down to -78°C, slowly add lithium diisopropylamide (35.8mL , 71.69mmol, 2M n-hexane solution), reacted at -78°C for 30 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com