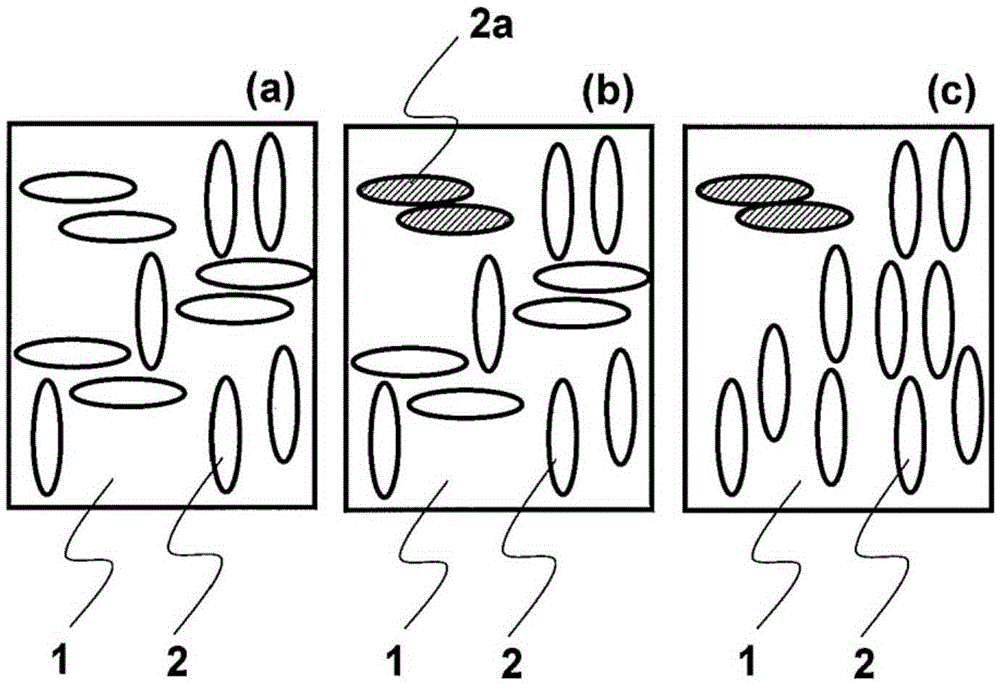
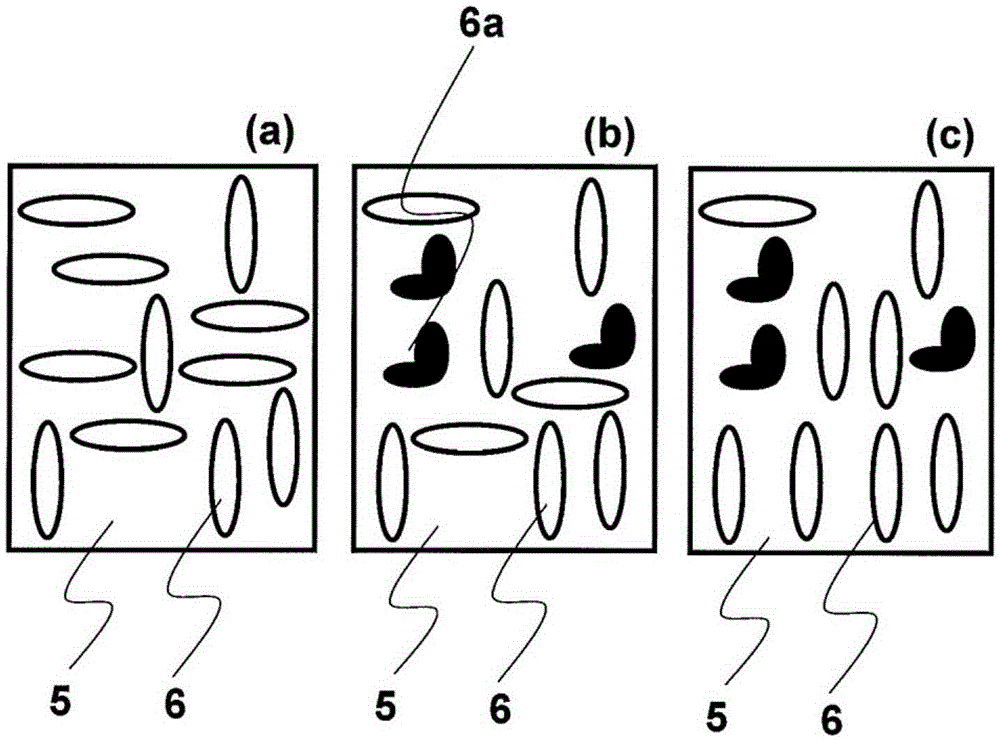
Method for producing substrate having liquid crystal orientation membrane for use in in-plane-switching liquid crystal display element
A group and alkyl technology, which is applied in the field of manufacturing liquid crystal display elements with excellent afterimage characteristics, can solve problems such as static electricity, dust generation, and inability to rub the liquid crystal alignment film evenly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0294] [Preparation of polymer composition]
[0295] The polymer composition used in the present invention is preferably prepared in the form of a coating liquid so as to be suitable for forming a liquid crystal aligning film. That is, the polymer composition used in the present invention is preferably prepared in the form of a solution in which a resin component for forming a resin coating is dissolved in an organic solvent. Here, the resin component refers to a resin component containing the above-mentioned photosensitive side chain type polymer capable of expressing liquid crystallinity. In this case, the content of the resin component is preferably 1% by mass to 20% by mass, more preferably 3% by mass to 15% by mass, particularly preferably 3% by mass to 10% by mass.
[0296] In the polymer composition of this embodiment, the above-mentioned resin components may all be the above-mentioned photosensitive side-chain polymers capable of exhibiting liquid crystallinity,...
Embodiment
[0390] The methacrylic monomers MA1 and MA2 used in Examples are shown below.
[0391] In addition, MA1 and M2 were respectively synthesized as follows. That is, MA1 was synthesized by the synthesis method described in the patent document (WO2011-084546). MA2 was synthesized by the synthesis method described in the patent document (Japanese Patent Laid-Open No. 9-118717).
[0392]
[0393] Crosslinking agents T1 to T14 used in Examples are shown below.
[0394] In addition, T1-T14 were synthesize|combined by the method shown below.
[0395] T1: diethoxy(3-glycidyloxypropyl)methylsilane (manufactured by Tokyo Chemical Industry Co., Ltd.).
[0396] T2: Tetraethoxysilane (manufactured by Tokyo Chemical Industry Co., Ltd.).
[0397] T3: N,N,N',N'-TETRAGLYCIDYL-4,4'-DIAMINODIPENYLMETHANE (N,N,N',N'-tetraglycidyl-4,4'-diaminodiphenylmethane, Aldrich system).
[0398] T4: Butanetetracarboxylic acid tetrakis(3,4-epoxycyclohexylmethyl) modified ε-caprolacton...
Synthetic example 1
[0418] After dissolving MA1 (9.97 g, 30.0 mmol) in THF (92.0 g) and degassing with a diaphragm pump, AIBN (0.246 g, 1.5 mmol) was added and degassed again. Then, it was made to react at 50 degreeC for 30 hours, and the polymer solution of the methacrylate was obtained. This polymer solution was added dropwise to diethyl ether (1000 ml), the resulting precipitate was filtered, washed with diethyl ether, and dried under reduced pressure in an oven at 40°C to obtain methacrylate polymer powder (A1) .
[0419] NMP (29.3g) was added to the obtained methacrylate polymer powder (A1) (6.0g), and it stirred and melt|dissolved at room temperature for 5 hours. NMP (24.7g) and BC (40.0g) were added and stirred to this solution, and the methacrylic polymer solution M1 was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com