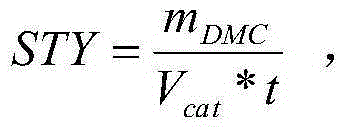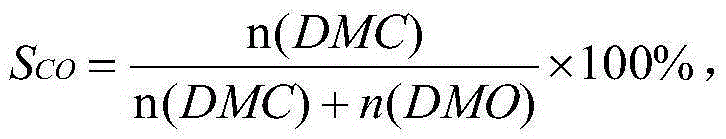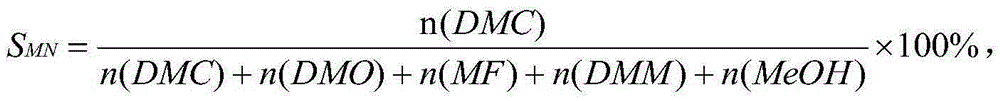Catalyst for synthesizing dimethyl carbonate through indirect vapor phase method and preparation method of catalyst
A technology of dimethyl carbonate and catalyst, which is applied in the field of catalyst and its preparation for gas-phase synthesis of dimethyl carbonate from CO and methyl nitrite, can solve the problems that catalyst activity and service life cannot be balanced, achieve high space-time yield, improve Dispersion, the effect of eliminating chlorine loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 0.17gPdCl 2 Dissolve with 15ml ammonia water, 0.54gCuCl 2 2H 2 O and 0.34g KCl were dissolved in 20ml of water. After the two were completely dissolved, they were mixed and diluted to 100ml with water, and the pH value was adjusted to 10 with ammonia water and dilute hydrochloric acid. This solution is slowly poured in the round-bottomed flask that has 10g through the NaY molecular sieve that has been treated with alkali, and magnetic force stirs 5 hours under 30 ℃, makes the metal cation in the main active component precursor and the assistant active component precursor and The cations in the carrier are fully exchanged. Then the temperature was raised to 80°C, and the ammonia in the solution was evaporated until the pH value was neutral. Suction filtration, washing, and drying at 110° C. for 6 hours to obtain the catalyst.
Embodiment 2
[0017] Take 0.25gPd(NO 3 ) 2 2H2O, add 15ml ammonia water, 0.75gCu(NO 3 ) 2 ·3H 2 Dissolve O in 20ml of water. After the two are completely dissolved, mix and dilute with water to 100ml. Use ammonia water and dilute hydrochloric acid to adjust the pH value to 10. This solution is slowly poured into the round-bottomed flask that has 10g through the NaY molecular sieve that has been treated with alkali, magnetically stirred 5 hours under 40 ℃, makes the metal cation in the main active component precursor and the assistant active component precursor and The cations in the carrier are fully exchanged. Then the temperature was raised to 90°C, and the ammonia in the solution was evaporated until the pH value was neutral. Suction filtration, washing, and drying at 110° C. for 6 hours to obtain the catalyst.
Embodiment 3
[0019] 0.255gPdCl 2 Dissolve with 15ml ammonia water, 0.81gCuCl 2 2H 2 Dissolve O in 20ml of water. After the two are completely dissolved, mix and dilute with water to 100ml. Use ammonia water and dilute hydrochloric acid to adjust the pH value to 11. This solution is slowly poured in the round-bottomed flask that has 10g through the NaX molecular sieve that has been treated with alkali, magnetically stirred 5 hours under 30 ℃, makes the metal cation in the main active component precursor and auxiliary active component precursor and The cations in the carrier are fully exchanged. Then the temperature was raised to 70°C, and the ammonia in the solution was evaporated until the pH value was neutral. Suction filtration, washing, and drying at 110° C. for 6 hours to obtain the catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com