A kind of antibacterial chitosan drug carrier and preparation method thereof
A chitosan and drug technology, applied in the field of degradable drug carrier and preparation, can solve the problems of changing the physical, chemical and biological properties of chitosan molecules, and achieve a solution with improved solubility, enhanced ability and good film-forming properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
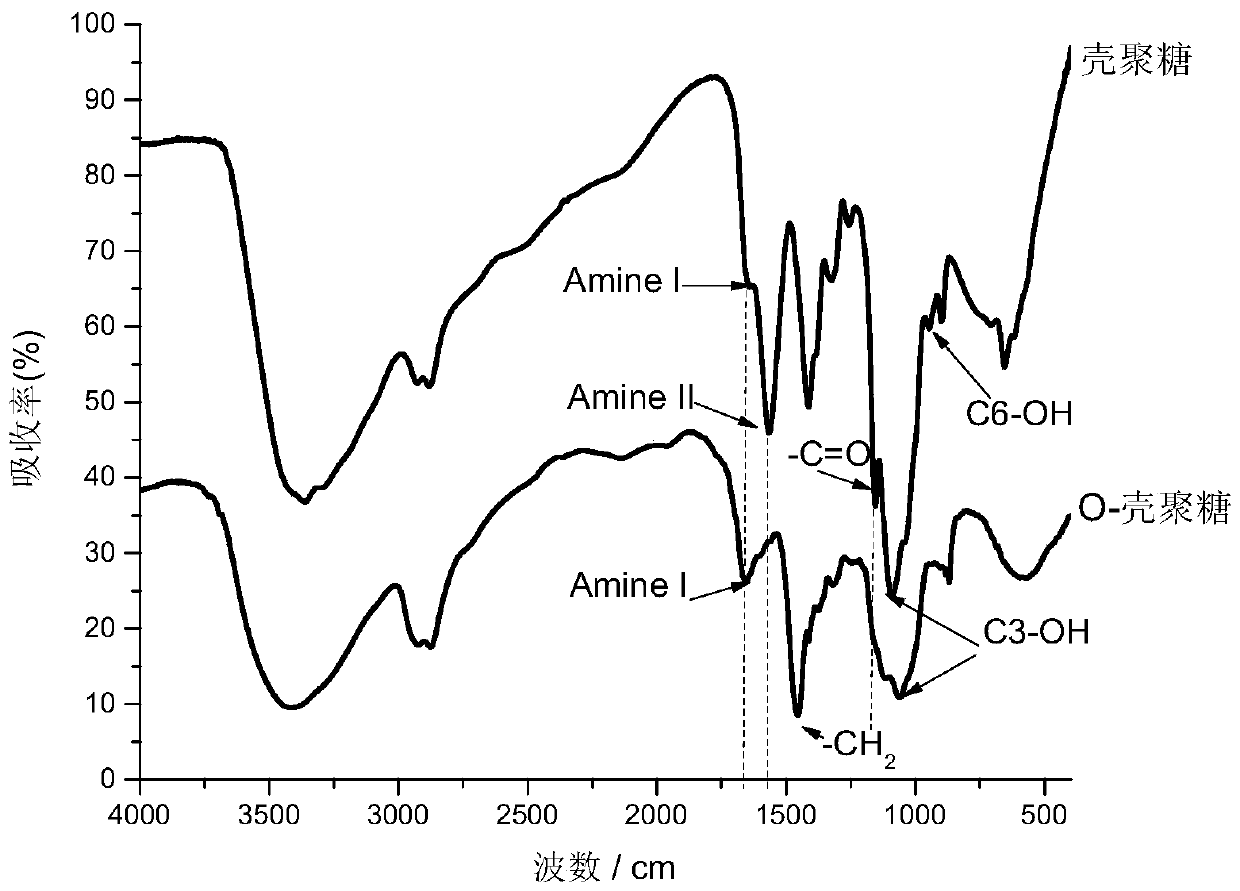
[0043] The preparation of embodiment 1 water-soluble O-chitosan
[0044] Put 5g of chitosan into a 500ml round bottom flask, add 100ml of dimethylformamide (DMF) and stir to swell for two hours, then add 10g of phthalic anhydride and stir thoroughly. Place the flask in an oil bath and rapidly raise the temperature to 130°C, keep it warm and stir for 12 hours. The product obtained is settled with acetone, washed twice with alcohol, washed once with ether, and dried with phosphorus pentoxide. Put the above-mentioned protected amine-based product into a 50ml beaker, add 30-50% (wt) sodium hydroxide solution (according to the ratio of 2-10ml sodium hydroxide solution added to 1g of the compound shown in formula II, after adding sodium hydroxide solution) After fully stirring, put it in a -20°C environment for 48 hours, take it out and squeeze out the excess sodium hydroxide after thawing, put the fixation into a 250ml round bottom flask, pour 30ml of isopropanol into it and stir w...
Embodiment 2
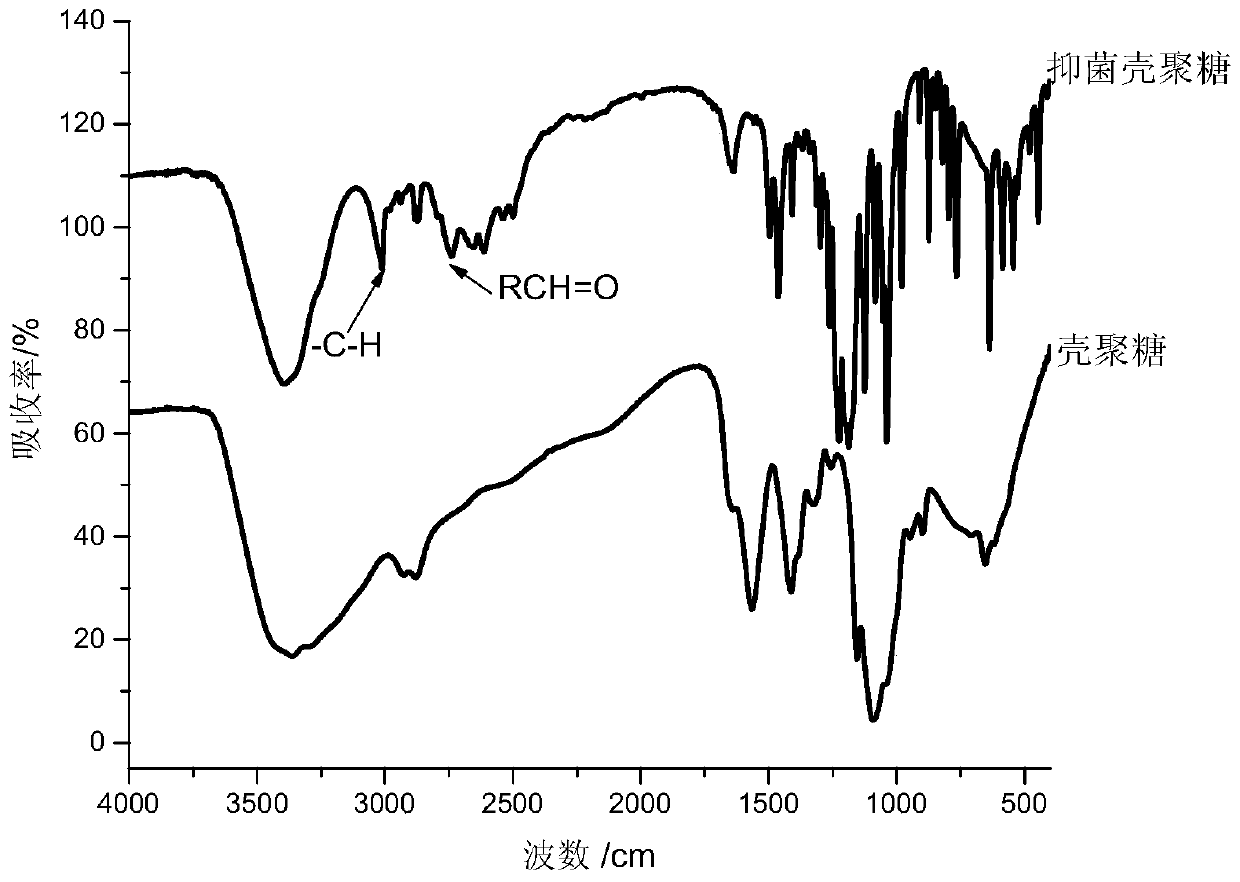
[0048] The preparation process of embodiment 2 antibacterial O-chitosan
[0049] For the preparation of acylated gallic acid, 1 g of anhydrous gallic acid was reacted with 1.8 g of acetyl chloride and 30 ml of dimethylformamide (DMF, as a catalyst) under reflux until the bubbles disappeared; excess acetyl chloride was evaporated, and a small amount of benzene was added for washing. Benzene and residual acetyl chloride were evaporated under reduced pressure, and volatile substances were removed from the product in a vacuum oven.
[0050] After dissolving the O-chitosan in Example 1 with a small amount of water, add 5 g of acetylated gallic acid, then add excess MES, NHS, EDC in turn, after stirring at room temperature for 36 h, filter; add excess tetrahydrofuran to the filtrate to settle , and then washed with a large amount of methanol, and the obtained product was alkalized with 15wt% NaOH for 8 hours, washed with methanol, and dried in vacuum to obtain the antibacterial O-ch...
Embodiment 3
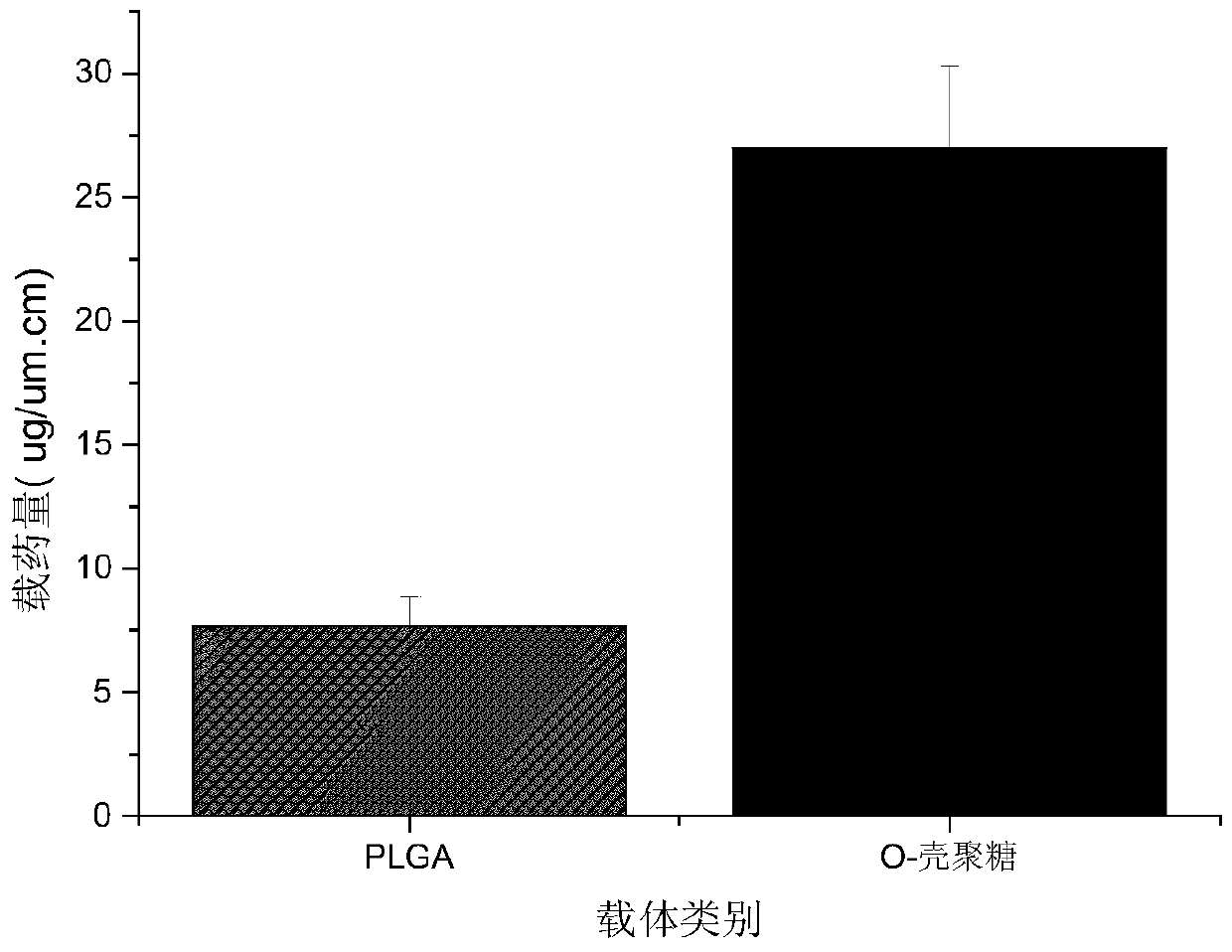
[0053] The specific application of embodiment 3 antibacterial chitosan drug carrier
[0054] The bacteriostatic chitosan solution obtained in Example 2 has a certain viscosity. In combination with the characteristics of ultrasonic atomization, the stent is first immersed in the medicated chitosan solution, and after taking out the filter paper to absorb excess liquid at one end, fix one end of the stent to the ultrasonic atomization solution. Put on the chemical equipment, start the program, put acetone into the drug inlet tube, the injection rate is 0.001-0.01ml / s, take it off after the surface of the stent is dry, and repeat it many times to obtain the drug coating with the designed thickness. Figure 4 is the drug release curve of the drug carrier coating, it can be seen that drug burst release occurred in the first six hours, and the average release rate was 13.35ug / day; due to the degradation characteristics of the antibacterial chitosan film, the drug was Hours later, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com