Method for separating terbutaline enantiomers in extracted mode through hydrophobicity phase transferring chirality
A terbutaline, hydrophobic technology, applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor solubility, excess enantiomeric content and low yield, etc., and achieves easy operation. , The effect of stable product quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
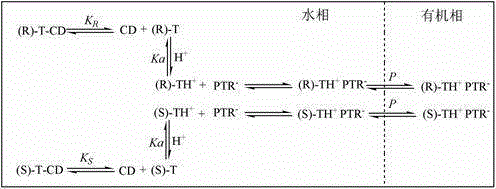
[0023] Take 0.5625g (0.0025mol) terbutaline enantiomer and dissolve it in 0.1mol / L Na 2 HPO 4 / NaH 2 PO 4 Adjust the pH to 7.0 in the buffer solution (0.1mol / L of hydroxypropyl-β-cyclodextrin), and prepare a 0.5L solution with a concentration of 0.005mol / L as the feed liquid;
[0024] Dissolve 441.3g (0.3mol) hydroxypropyl-β-cyclodextrin in 0.1mol / L Na 2 HPO 4 / NaH 2 PO 4 Adjust the pH to 7.0 in the buffer solution to prepare a 3L solution with a concentration of 0.1mol / L as the water phase;
[0025] Dissolve 20.52g (0.06mol) of sodium tetraphenylborate in an organic solvent (dichloromethane, 1,2-dichloroethane, n-hexane, n-heptane, cyclohexane, n-octanol, n-heptanol). 3L organic solvent with a concentration of 0.02mol / L as the organic phase;
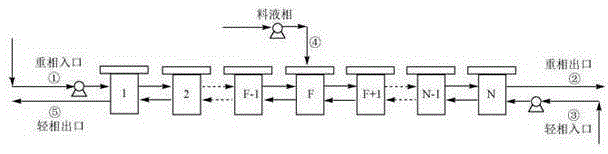
[0026] Connect 10-stage centrifugal extractors in series. Use a constant flow pump to first pump the heavy phase into the centrifugal extractor. When there is a solution flowing out of the heavy phase outlet, pump the light phase from the corr...
Embodiment 2
[0028] Take 0.5625g (0.0025mol) terbutaline enantiomer and dissolve it in 0.1mol / L Na 2 HPO 4 / NaH 2 PO 4 Adjust the pH (5.0, 6.0, 7.0, 8.0, 9.0) in the buffer solution (0.1mol / L for hydroxypropyl-β-cyclodextrin), and prepare a 0.5L solution with a concentration of 0.005mol / L as the feed solution phase;
[0029] Dissolve 441.3g (0.3mol) hydroxypropyl-β-cyclodextrin in 0.1mol / L Na 2 HPO 4 / NaH 2 PO 4 Adjust the pH (5.0, 6.0, 7.0, 8.0, 9.0) in the buffer solution to prepare a 3L solution with a concentration of 0.1mol / L as the water phase;
[0030] Take 20.52g (0.06mol) of sodium tetraphenylborate dissolved in n-octanol to form a 3L organic solvent with a concentration of 0.02mol / L as the organic phase;
[0031] Connect 10-stage centrifugal extractors in series. Use a constant flow pump to first pump the heavy phase into the centrifugal extractor. When there is a solution flowing out of the heavy phase outlet, pump the light phase from the corresponding inlet into the centrifugal extra...
Embodiment 3
[0033] Dissolve 0.5625g (0.0025mol) terbutaline enantiomer in 0.1mol / L Na 2 HPO 4 / NaH 2 PO 4 Adjust the pH to 7.0 in the buffer solution (0.1mol / L of hydroxypropyl-β-cyclodextrin), and prepare a 0.5L solution with a concentration of 0.005mol / L as the feed liquid;
[0034] Dissolve 441.3g (0.3mol) hydroxypropyl-β-cyclodextrin in 0.1mol / L Na 2 HPO 4 / NaH 2 PO 4 Adjust the pH to 7.0 in the buffer solution to prepare a 3L solution with a concentration of 0.1mol / L as the water phase;
[0035] Dissolve the phase transfer agent (sodium tetraphenylborate, potassium tetraphenylborate, sodium hexafluorophosphate, potassium hexafluorophosphate) in n-octanol to prepare a 3L organic solvent with a concentration of 0.02mol / L as the organic phase;
[0036] Connect a 10-stage centrifugal extractor in series, use a constant flow pump to first pump the heavy phase into the centrifugal extractor. When the heavy phase exits the solution, pump the light phase into the centrifugal extractor from the corre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com