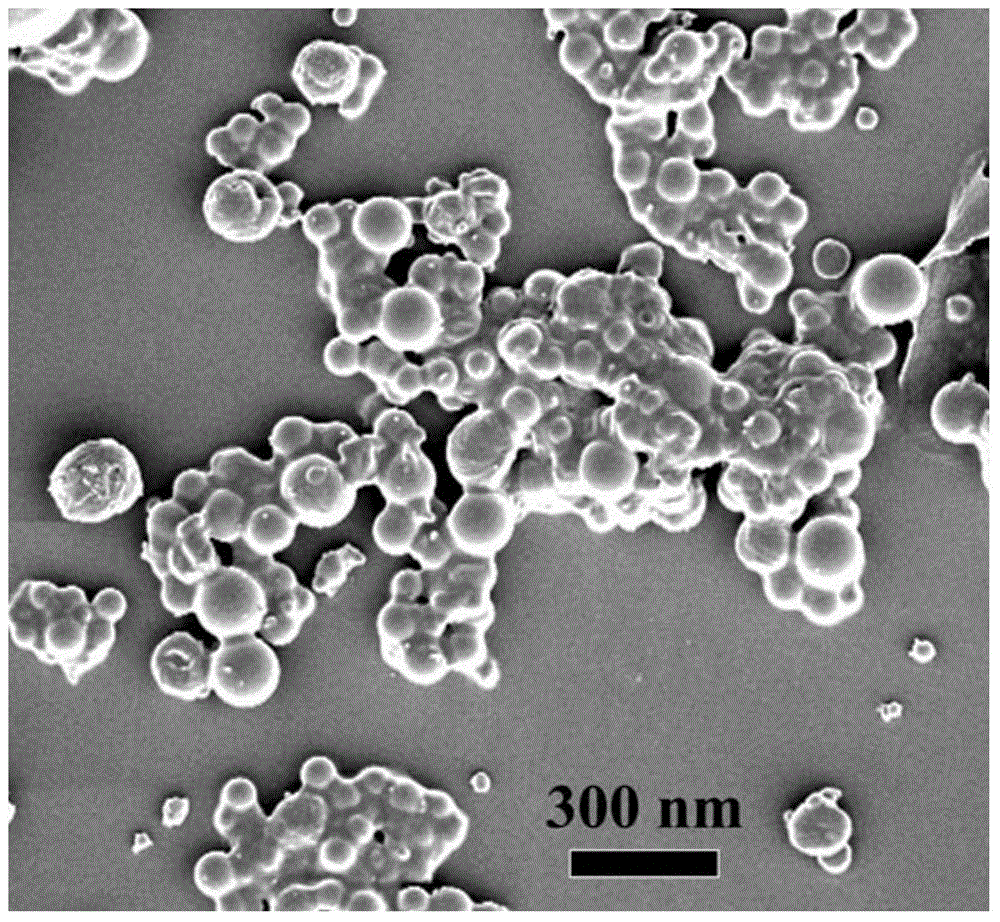
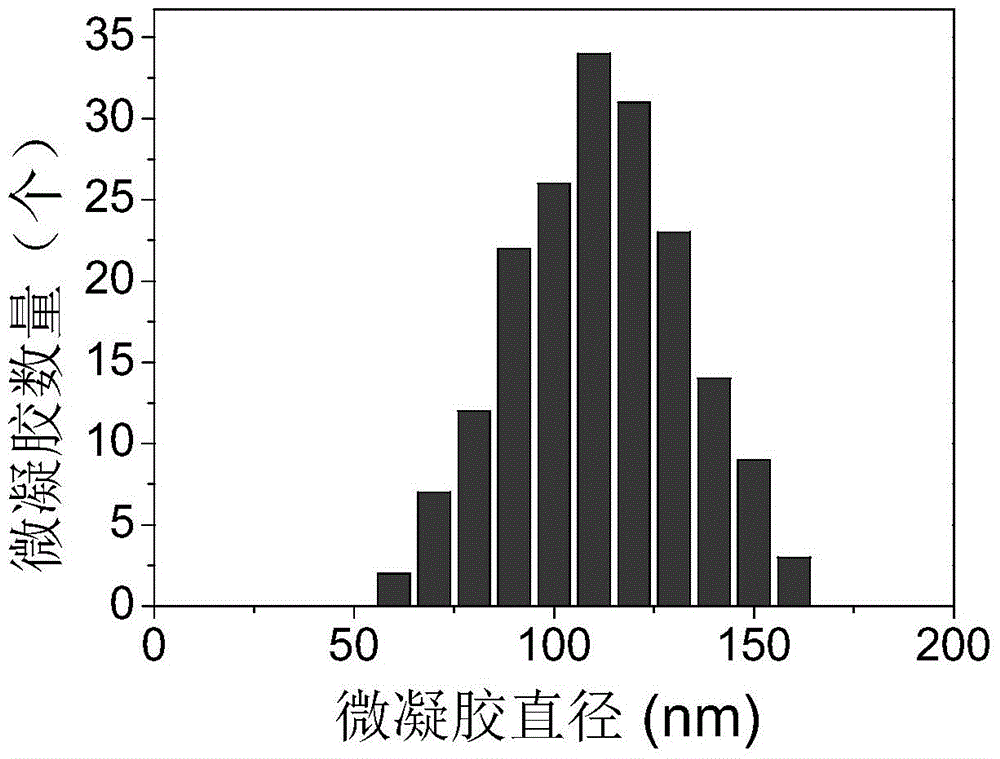
Magnetic polysaccharide nanometer gel material preparation method
A nanogel, polysaccharide technology, applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problem of low magnetic flux of polysaccharide nano or microsphere materials, difficult to achieve targeted delivery of drugs, difficult to meet clinical use, etc. problems, to achieve the effect of eliminating toxic residues, good positioning effect, and large drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The method for preparing magnetic polysaccharide nanogel provided by the present invention has specific steps including:
[0044] Step 1. Prepare an aqueous solution of heparin with a mass / volume concentration of 0.6-2% at room temperature, and add dropwise a thymine hydrochloric acid solution with a mass concentration of 0.1-0.2%, the volume of which is 1 / 5 to 1 / 2 of the heparin solution. Stir the reaction at ℃~80℃ for 12~24 hours, adjust the reaction solution to neutral with ammonia water, then extract with ethyl acetate, and evaporate to dryness to obtain the basicized heparin;
[0045] Step 2. Prepare a chitosan aqueous solution with a mass / volume concentration of 0.3-1% at room temperature, and add dropwise adenine hydrochloric acid solution with a mass concentration of 0.1-0.2%, whose volume is 1 / 10-1 / of the chitosan solution 5. Stir the reaction at 50°C to 80°C for 12 to 24 hours, adjust the reaction solution to neutral with ammonia water, then extract with ethyl ace...
Embodiment 1
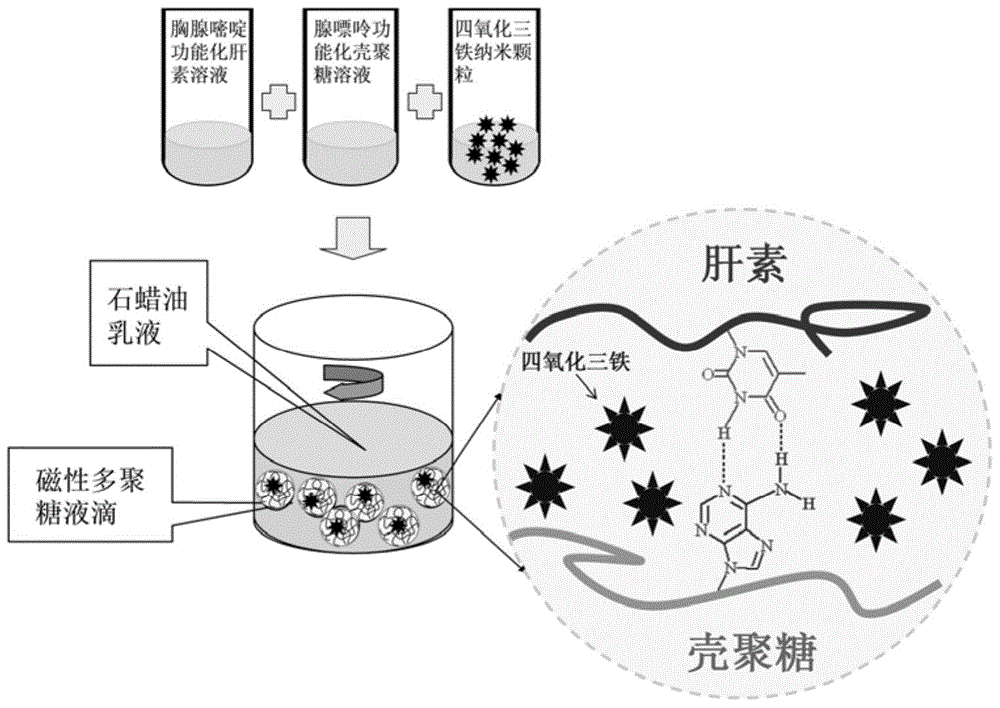
[0050] (1) Prepare a 2% heparin aqueous solution with a mass / volume concentration at room temperature, add dropwise a 0.2% mass / volume concentration of thymidine hydrochloric acid solution, whose volume is 1 / 2 of the heparin solution, and stir for 24 hours at 50°C. , Adjust the reaction solution to neutral with ammonia water, then extract with ethyl acetate, and evaporate to dryness to obtain alkalinized heparin. The preparation diagram is shown in figure 1 ;
[0051] (2) Prepare chitosan aqueous solution with a mass / volume concentration of 0.5% at room temperature, and add dropwise adenine hydrochloric acid solution with a mass / volume concentration of 0.2%, whose volume is 1 / 5 of the chitosan solution, at 50°C The reaction was stirred for 24 hours, the reaction solution was adjusted to neutral with ammonia water, and then extracted with ethyl acetate and evaporated to dryness to obtain alkalized chitosan. The preparation diagram is shown in figure 1 ;
[0052] (3) Dissolve ferrous...
Embodiment 2
[0056] (1) Prepare a 0.6% heparin aqueous solution with a mass / volume concentration of 0.6% at room temperature, add dropwise a 0.1% mass / volume concentration of thymine hydrochloric acid solution whose volume is 1 / 5 of the heparin solution, and stir for 12 hours at 60°C. , Adjust the reaction solution to neutral with ammonia water, then extract with ethyl acetate, and evaporate to dryness to obtain basic heparin;
[0057] (2) Prepare an aqueous solution of chitosan with a mass / volume concentration of 0.3% at room temperature, and add dropwise adenine hydrochloric acid solution with a mass / volume concentration of 0.1%, whose volume is 1 / 6 of the chitosan solution, at 60°C The reaction was stirred for 12 hours, the reaction solution was adjusted to neutral with ammonia water, and then extracted with ethyl acetate and evaporated to dryness to obtain alkalized chitosan;
[0058] (3) Dissolve ferrous chloride and ferric chloride in water at room temperature, their molar mass ratio is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com