Antipyretic analgesic anti-inflammatory aspirin composition tablet
A technology of aspirin and anti-inflammatory drugs, applied in the field of medicine, can solve the problems of inconvenient medication, salicylic acid poisoning reaction, gastrointestinal bleeding, etc., achieve low free salicylic acid content, and reduce the effect of gastrointestinal adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of aspirin crystals
[0030] (1) Grind the crude aspirin, pass it through a 60-mesh sieve, and then add it to a mixed solution of ether and chloroform whose volume is 8 times the weight of aspirin. The volume ratio of ether and chloroform is 2:3, 130 rpm / min stirring for 10 minutes;
[0031] (2) Add ethanol with a volume 3 times the weight of aspirin under stirring at 90 rpm, and raise the temperature to 30°C at the same time;
[0032] (3) After adding the solution, let it stand still for 3 hours, add distilled water at 0°C with a volume 8 times the weight of aspirin dropwise under the condition of stirring at 150 rpm, and finish adding dropwise at a uniform speed within 2 hours;
[0033] (4) After the dropwise addition, cool down to -15°C, continue to stir at a stirring rate of 110 rpm for 2 hours, let stand for 1 hour to precipitate crystals, filter, wash, and vacuum dry to obtain aspirin crystals.
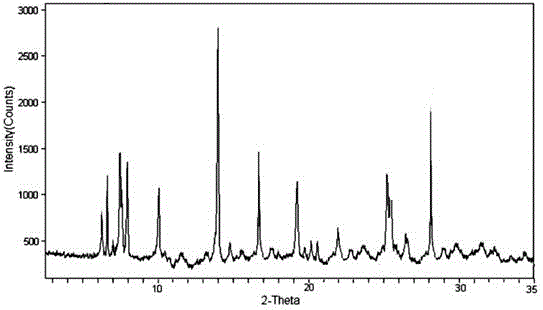
[0034] The X-ray powder diffraction pat...
Embodiment 2
[0035] Example 2: Preparation of aspirin tablets
[0036] Prescription: 5 parts by weight of aspirin prepared in Example 1, 7.3 parts by weight of microcrystalline cellulose, 5.4 parts by weight of lactose, 3 parts by weight of sodium starch glycolate, 0.15 parts by weight of nicotinamide, and 0.3 parts by weight of povidone K30, 4 parts by weight of 95% ethanol, and 0.4 parts by weight of magnesium trisilicate.
[0037] Preparation:
[0038] (1) Processing of raw and auxiliary materials: sieve aspirin to 100 mesh, sieve carboxymethyl starch sodium and nicotinamide to 80 mesh;
[0039] (2) Weighing: Weighing according to the prescription;
[0040] (3) Adhesive preparation: dissolve the prescribed amount of povidone K30 in 95% ethanol and set aside;
[0041] (4) Granulation: Add aspirin, microcrystalline cellulose, lactose, carboxymethyl starch sodium, and nicotinamide into the wet mixing granulator, dry mix for 10 minutes, add the prepared binder, wet mix and cut 150 -18...
Embodiment 3
[0046] Example 3: Preparation of aspirin tablets
[0047] Prescription: 5 parts by weight of aspirin prepared in Example 1, 7.4 parts by weight of microcrystalline cellulose, 5.5 parts by weight of lactose, 4 parts by weight of sodium starch glycolate, 0.2 parts by weight of nicotinamide, and 0.4 parts by weight of povidone K30, 5 parts by weight of 95% ethanol, and 0.5 parts by weight of magnesium trisilicate.
[0048] Preparation:
[0049] (1) Processing of raw and auxiliary materials: sieve aspirin to 100 mesh, sieve carboxymethyl starch sodium and nicotinamide to 80 mesh;
[0050] (2) Weighing: Weighing according to the prescription;
[0051] (3) Adhesive preparation: dissolve the prescribed amount of povidone K30 in 95% ethanol and set aside;
[0052] (4) Granulation: Add aspirin, microcrystalline cellulose, lactose, carboxymethyl starch sodium, and nicotinamide into the wet mixing granulator, dry mix for 10 minutes, add the prepared binder, wet mix and cut 150 -180...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com