Preparation method of vinpocetine freeze-dried powder for injection
A technology of vinpocetine and freeze-dried powder, which is applied in the direction of freeze-dried transportation, making drugs into special physical or taking forms, powder transportation, etc., which can solve the problem of unstable quality of vinpocetine freeze-dried powder for injection, Parameter control is not accurate enough, the process is not very standardized, etc., to achieve the effect of improving the safety of clinical use, standardization of the preparation process, and high safety of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
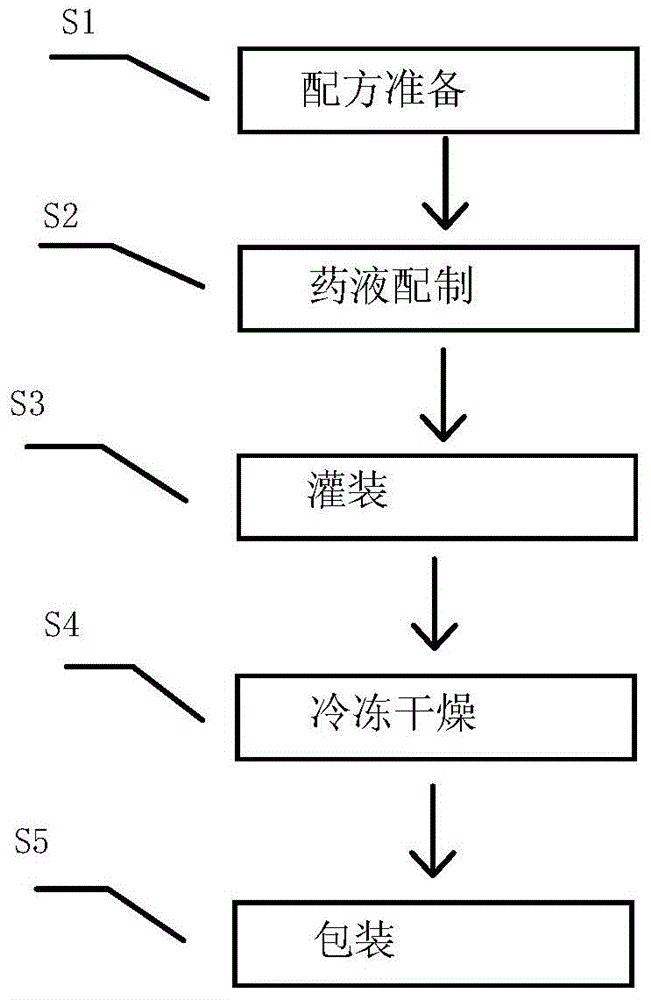
[0034] Such as figure 1 As shown in the flow chart, according to the method provided by the present invention, a method for preparing vinpocetine freeze-dried powder for injection includes the steps: S1 formula preparation, S2 liquid medicine preparation, S3 filling, S4 freeze-drying and S5 packaging, wherein ,
[0035] S1 recipe preparation
[0036] For every 1,000 pieces of the vinpocetine freeze-dried powder pharmaceutical composition for injection, the formula is as follows:
[0037]
[0038] The formula of the invention is simple, the auxiliary materials are less, the side effects caused by excessive addition of auxiliary materials are avoided, and the safety of clinical use is improved.
[0039] S2 liquid medicine preparation:
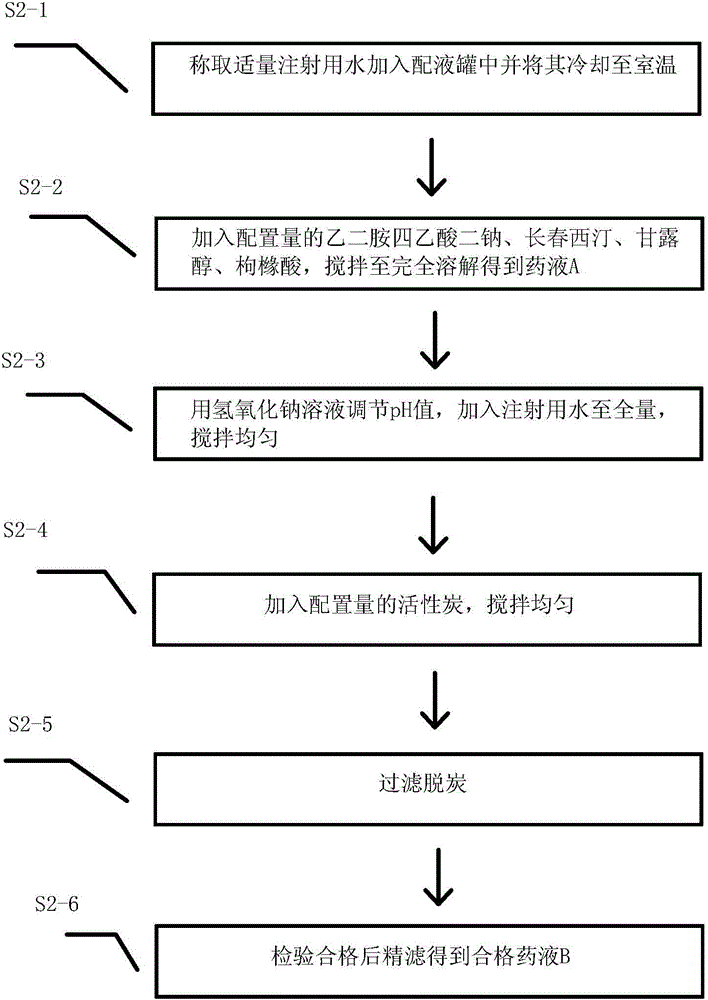
[0040] Such as figure 2 As shown in the flowchart, it includes the following steps
[0041] S2-1. Weigh an appropriate amount of water for injection into the mixing tank and cool it to room temperature. The amount of water for injection added for the firs...
Embodiment 2 3
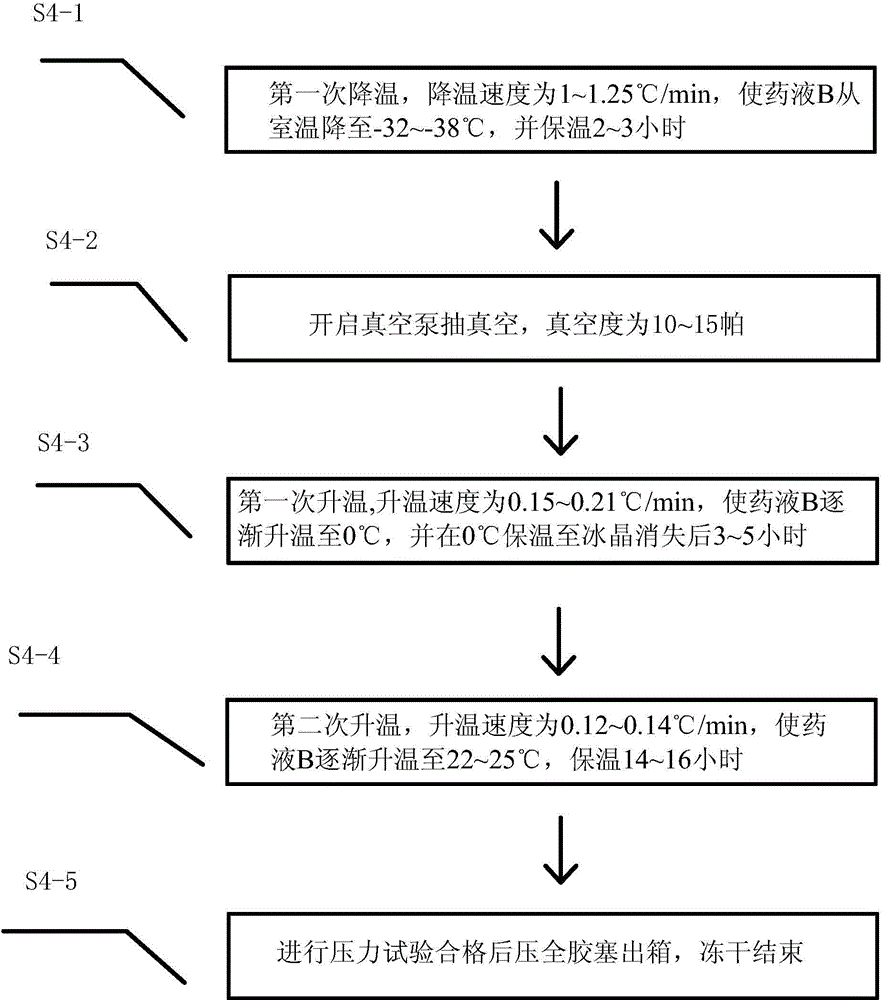
[0062] The first time injection water consumption in step S2-1, the pH value in S2-3, the first cooling down, the first heating up, the second heating up speed, and the holding temperature in steps S4-1 to S4-4 , The holding time and the degree of vacuum are replaced with the values shown in Table 1, and the stability study of the finished product at room temperature is continued. The results are shown in Table 1. Compared with the prior art, the method of the present invention uses vacuum The low-temperature freeze-drying process ensures that the medicine is not easily oxidized and deteriorated, and overcomes the problem of decomposition of the medicine due to high temperature in the production process. The obtained vinpocetine freeze-dried powder for injection has high quality, good stability, and water content Low, extending the shelf life.
[0063] Table 1 The shelf life of vinpocetine freeze-dried powder for injection prepared under different conditions
[0064]
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com