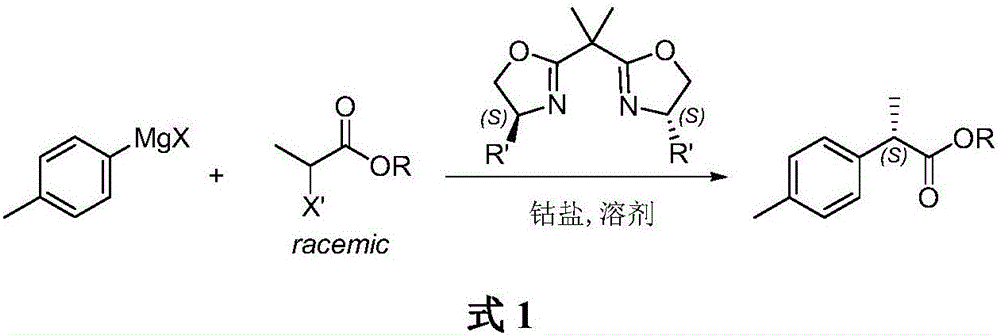
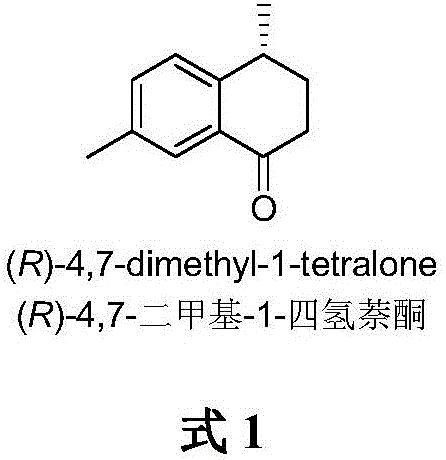
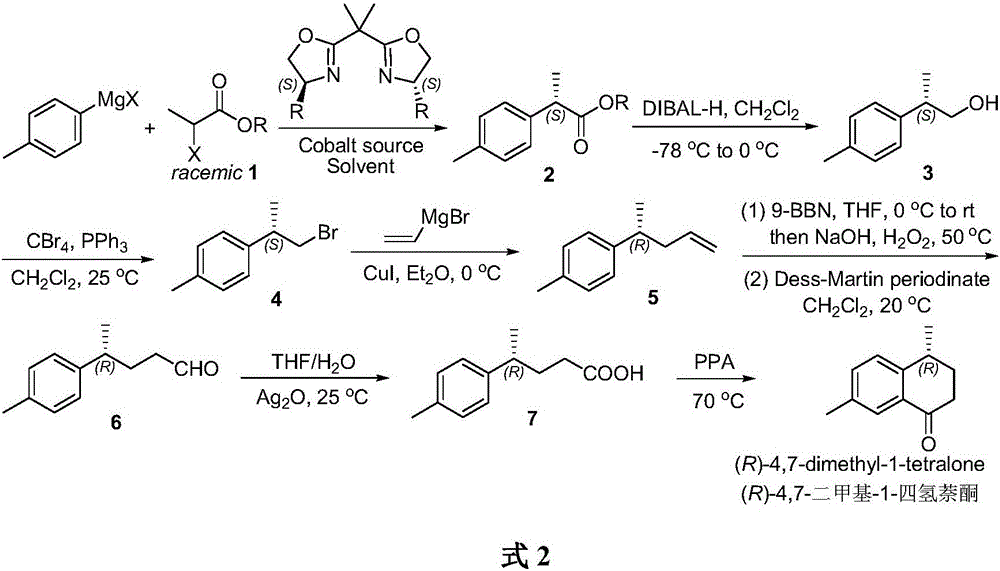
A kind of method of asymmetric catalytic synthesis (r)-4,7-dimethyl-1-tetralone
A technology of tetralone and dimethyl, which is applied in the field of new asymmetric catalytic synthesis of -4,7-dimethyl-1-tetralone, which can solve the problems of limiting biological activity research and synthetic application research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of (S)-benzyl p-toluene propionate 2
[0024] Under the protection of argon, quickly weigh anhydrous CoI 2 (0.63g, 2mmol) in a 200mL Schlenk reaction flask, vacuum-dried for 30min. After cooling to room temperature, add (4S,4'S)-bis(4-benzyloxazolin-2-yl)-propane L1 (0.87g, 2.4mmol), inject anhydrous tetrahydrofuran (15mL) with a syringe, and stir at room temperature 1h. Then racemic benzyl 2-bromophenylpropionate (4.86 g, 20 mmol) was added, and after the addition was complete, the temperature of the mixture was lowered to -78°C. p-Tolylmagnesium bromide (30mL, 1.0M THF solution, 30mmol) was added slowly, and the stirring reaction was continued at -78°C for 8h. After the reaction was completed, saturated ammonium chloride aqueous solution (20 mL) was added to quench the reaction, and the organic layer was separated. The aqueous layer was extracted with ether (3 x 20 mL), and the organic layers were combined and washed with saturated aqueous sodium chlorid...
Embodiment 2
[0028] Synthesis of (S)-p-tolylpropanol 3
[0029] Under the protection of argon, inject anhydrous CH with a syringe 2 Cl 2 (25mL) was injected into a 100mL Schlenk reaction flask, and (S)-benzyl p-toluenepropionate 2 (3.81g, 15mmol) was slowly added. The temperature of the mixture was lowered to -78°C, and diisobutylaluminum hydride (DIBAL-H) (22 mL, 1.5M solution in toluene, 33 mmol) was slowly added dropwise using a syringe pump. At this temperature, the reaction was stirred for 30 minutes, then the temperature was raised to 0° C., and the reaction was stirred for another 30 minutes. After the reaction, the temperature of the reaction solution was lowered to -78°C, and the reaction was quenched with methanol (1 mL). Then, an aqueous potassium sodium tartrate solution (66 mL, 0.5 M, 33 mmol) was added, the temperature of the mixture was raised to room temperature, and stirring was continued for 12 h. Separate the organic layer and the aqueous layer with CH 2 Cl 2 (3 ...
Embodiment 3
[0031] Synthesis of (S)-2-p-tolyl-1-bromopropane 4
[0032] Add (S)-p-tolylpropanol 3 (1.50g, 10mmol) and triphenylphosphine (3.15g, 12mmol) into a 100mL reaction flask, then add CH 2 Cl 2 (33mL), stir well. Add CBr slowly 4 (3.42g, 10.3mmol), the temperature of the reaction solution was raised to room temperature, and the stirring reaction was continued for 4h. Concentrate under reduced pressure to remove the solvent, add petroleum ether (40 mL) to the residue, filter with suction, wash the filter residue with petroleum ether, and combine the filtrates. with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was purified by silica gel column chromatography (petroleum ether) to obtain (S)-2-p-tolyl-1-bromopropane 4 (2.01g, yield 94%, optical purity 92%) in light yellow oily liquid . [α] D 20 =-22.7(c 1.3, CHCl 3 ). 1 H NMR (300MHz, CDCl 3 )δ7.15(d, J=8.3Hz, 2H), 7.11(d, J=8.3Hz, 2H), 3.58(dd, J=9.8,6.1Hz, 1H), 3.46(dd, J=9.8,8.0 Hz,1H),3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com